- Indomethacin also known as indometacin is NSAIDS (Non- Steroidal Anti-Inflammatory Drugs) and is potent analgesic, anti-pyretic and anti-inflammatory drug.
- It is indole acetic acid derivative, was patented in 1961 and was approved for medical use in 1963.
- Indomethacin was among the first NSAIDs to be used in treatment of migraine and for headaches that was later on known as “indomethacin-responsive” headache disorders.
Mechanism of action of Indomethacin
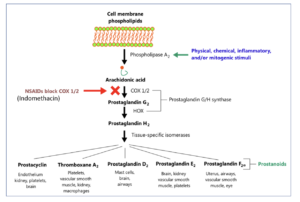
Figure 1- Mechanism of action of Indomethacin
- It is non-selective inhibitor of cyclooxygenase enzymes (COX-1 and COX-2) which participate in synthesis of prostaglandins from arachidonic acid.
- During pain, inflammation and fever, arachidonic acid is liberated from phospholipid fraction of cell membrane by phospholipase A2. Arachidonic acid is converted to prostaglandins (PGs) by cyclooxygenase (COX-1 and COX-2).
- The prostaglandins produced sensitize blood vessels to other inflammatory mediators which increase permeability and sensitize chemical receptor of afferent pain ending to mediators such as histamine and bradykinin. PGE2 and PGI2 produce hyperalgesia associated with inflammation. They are also involved in pyretic response.
- This inhibition by indomethacin is responsible for its analgesic, anti-pyretic and anti-inflammatory action. The inhibition is reversible once serum drug level decline after discontinuation of therapy.
Pharmacokinetics of Indomethacin
- It can be administered through oral, rectal, vaginal or systemic route.
- Following oral administration, it is absorbed rapidly and completely. Peak plasma concentration is achieved within 1-2 hours. Ingestion of food may reduce and delay the peak concentration without reducing the amount absorbed.
- About 90% of indomethacin bound to plasma protein; albumin at therapeutic plasma concentration. It is distributed in synovial fluid, can cross placental barrier in significant amount and is secreted in breast milk.
- It is mainly metabolized in liver and its metabolites are O-desmethylindomethacin, N-deschlorobenzoylindomethacin and O-desmethyl-N-deschlorobenzoylindomethacin. The metabolites don’t possess anti-inflammatory activity.
- About 50-60 % of an oral dose is excreted in urine within 24 hours in glucuronidated from. Remaining 40 % is excreted in feces after biliary secretion.
- Its biological half-life is about 5 to 10 hours and a plasma clearance is about 1 to 2.5ml/kg/min. Its serum concentration is not affected by renal failure.
Therapeutic Uses of Indomethacin
- It is effective in relieving joint pain, swelling, tenderness and decreasing duration of morning stiffness. It is estimated to be about 20 times more potent than aspirin.
- In patients with rheumatoid arthritis, it is useful to relieve joint pain and reduction in joint swelling.
- To treat acute gout pain.
- It helps to reduce pain even in absence of clinically obvious inflammation, e.g. ankylosing spondylitis.
- In osteoarthritis and gouty arthritis.
- To treat shoulder pain caused by bursitis or tendonitis.
- To treat persistent patent ductus arteriosus in pre-term infants.
Adverse effects
- The most common side effects are CNS related side effects like severe frontal headache (occurring in 25-50% of patients taking drug for long time), followed by giddiness, mental confusion, blurred vision, depression and psychotic disturbance. These effects may hinder in patient’s ability to drive vehicle. Such neuropsychiatric effects are more common with indomethacin than other NSAIDs.
- Other common side effects are gastrointestinal complains like diarrhea, nausea, vomiting, dyspepsia, bleeding in stomach and possibility of peptic ulcer.
- It may cause sodium retention, edema and nephrotoxicity.
- Hematopoietic reactions include neutropenia, thrombocytopenia and rarely aplastic anemia.
- It increases risk of heart attack or stroke.
- Acute pancreatitis may occur rarely.
Drug Interaction
- When used concurrently with lithium, it increases serum concentration of lithium by decreasing its renal clearance. Thus, it increases chance of lithium toxicity and hence avoided in patients on lithium therapy.
- It also increases serum concentration of aminoglycosides and methotrexate.
- Cholestyramine and colestipol may decrease absorption of indomethacin.
- Concurrent administration with aspirin increases chance of developing ulcer. If taken with warfarin or other oral anti-coagulants, risk of bleeding may increase.
- It reduces effect of furosemide and thiazide diuretics and increases negative effect of cyclosporine on kidney function.
- It can interact with probenecid, heart or blood pressure medication like ACE inhibitors, ARBs (Angiotensin Receptor Blocker), beta-blockers and digoxin.
Contraindication
- It is avoided in patients with
- Concurrent peptic ulcer or history of ulcer.
- Allergic to indomethacin or other NSAIDs.
- Renal and liver damage.
- In children under 2 years of age (except in neonates with patent ductus arteriosus).
- It should be avoided in individual who recently had a heart attack. It should be avoided before or after heart bypass surgery.
- Should be used with caution in elderly patients with Parkinson’s disease, epilepsy or psychiatric disorder as there remains greater risk of developing serious CNS adverse effects.
- It should be avoided in patients with a history of asthma attack.
References
- https://medlineplus.gov/druginfo/meds/a681027.html
- Lucas S. The Pharmacology of Indomethacin. Headache the Journal of Head and Face Pain. 2016; 56(2): 436-446.
- Helleberg L. Clinical Pharmacokinetics of Indomethacin. Clinical Pharmacokinetics. 1981; 6: 245-258.
- Pacifici GM Clinical Pharmacology of Indomethacin in Preterm Infants: Implications in Patent Ductus Arteriosus Closure. Paediatr Drugs. 2013; 15(5): 363-76.
- Clyman R, Wickremasinghe A, Jhaveri N, Hassinger DC, Attridge JT, Sanocka U et al. Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus. J Pediatr. 2013; 163(2): 406–411.e4.
- Goodman and Gillman’s Manual of Pharmacology.
- Pharmacology and pharmacotherapeutics. 24th edition.
Very informative and educative
Thank you. please, keep visiting our website.