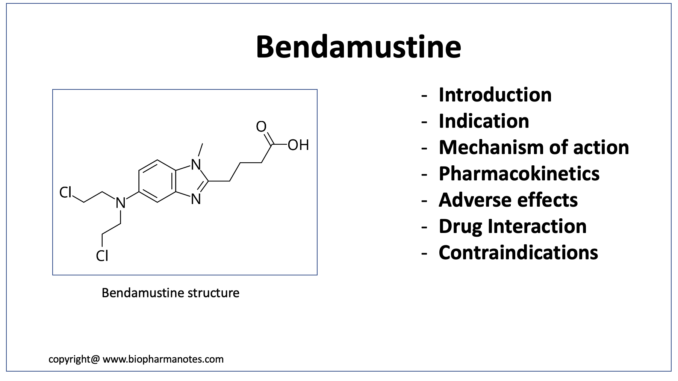
- Bendamustine is unique structured cytotoxic alkylating agent. Chemically, it is 4-[5-[bis(2-chloroethyl)amino]-1-methyl-benzoimidazol-2-yl] butyric acid hydrochloride. It is a nitrogen mustard drug. Bendamustine consist of three major components in its structure: mechlorethamine group with alkylating properties, benzimidazole ring that may exert antimetabolite activity and butyric acid side chain that increases water solubility. Bendamustine was synthesized in 1963 by Ozegowski and Krebs at the Institute for Microbiology and Experimental Therapy in Jena, in German Democratic Republic (East Germany).
- Its systematic study was not done until the 1990s. It received its first marketing approval in Germany, under the tradename Ribomustin, for use as a single-agent or in combination chemotherapy regimens for indolent NHL, multiple myeloma (MM), and chronic lymphocytic leukemia (CLL).
- Bendamustine got its FDA approval in March 2008. It is included in World Health organization’s List of Essential Medicines.
Indications of Bendamustine
- Used for treatment of chronic lymphocytic leukemia.
- For treatment of indolent B cell non-Hodgkin Lymphoma which has progressed following rituximab treatment and in mantle cell lymphoma. It is used in combination with anti CD20 monoclonal antibodies in these lymphomas.
Mechanism of action of Bendamustine
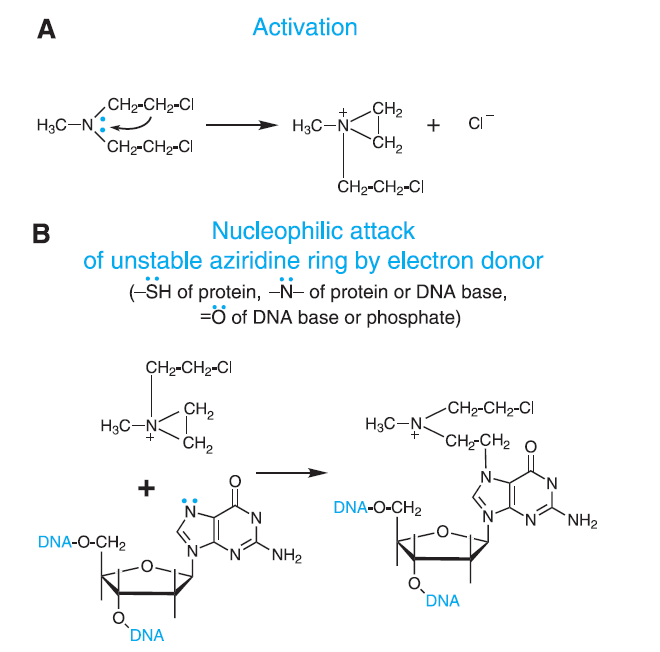
Figure- Figure 1- Mechanism of action of Alkylating Agents (Source- Goodman and Gillman Book)
- Its exact and detailed mechanism of action is not known. It is a bifunctional mechlorethamine derivative and can from electrophilic alkyl group that form covalent bond with other molecules. This leads to formation of intra and inter strand cross linking between bases of DNA and results in cell death.
- It is active against both active and quiescent cells. It causes DNA breaks which are more extensive than those produced by cyclophosphamide or carmustine and more durable than those caused by melphalan, cyclophosphamide, or carmustine. Compared to other alkylating agents, DNA damage caused by bendamustine may undergo relatively slower repair process.
Pharmacokinetics of bendamustine
- It is administered via IV route and is available as solution or lyophilized powder. Binding to plasma protein is high (around 94-96%). However, there is less chances of its displacement by other high protein bound drugs. Only its free form is active and exert its effect.
- Its metabolism takes place in liver. Some of its metabolites are mono-hydroxy bendamustine (HP1) and di-hydroxy bendamustine (HP2), g-hydroxy bendamustine (M3) and N-desmethyl-bendamustine (M4). HP1 and HP2 have little or no activity and are product of hydrolysis of mechlorethamine group. M3 and M4 are active metabolites. M3 is product of g oxidation of butyric acid side chain and M4 is product of demethylation of benzimidazole ring.
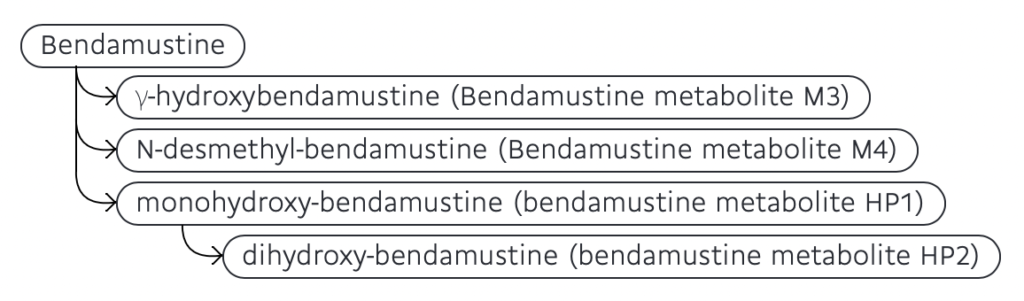
Figure- Metabolites of bendamustine (Source- Drug bank online)
- Renal or hepatic function has less effect on bendamustine exposure due to its short half-life and metabolic pathways. Hence, dosage adjustment is not required in mild to moderate impairment of liver and kidney.
Adverse effects
- When compared to other combination of chemotherapeutic agents, it has less adverse effects. Some common side effects include nausea, vomiting, skin rashes, myelosuppression, fatigue etc.
- There are some rare cases of anaphylaxis or infusion reaction.
Drug Interaction
- It shows synergistic effect when used with rituximab.
Contraindications/ Warning
- Contraindicated in patients allergic to bendmaustine or any of ingredients in the formulation.
- In 2015, FDA issued a warning to not use bendmaustine solution with closed system transfer devices, adapter and syringes which contain polycarbonate or acrylonitrile-butadiene-styrene. This is because, bendamustine injection contains N, N-dimethyl acetamide (DMA) which is incompatible with polycarbonate and acrylonitrile-butadiene-styrene.
References
- Tageja N. Bendamustine: Safety and Efficacy in the Management of Indolent Non-Hodgkins Lymphoma. Clinical Medicine Insights: Oncolog. 2011:5 145–156.
- Dogliotti I et al. Real-Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia. J. Pers. Med. 2021; 11(4): 249.
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-against-using-treanda-injection-solution-closed-system-transfer-devices-adapters-and
- https://go.drugbank.com/drugs/DB06769