- Losartan belongs to class of drug called ARBs (Angiotensin Receptor Blocker) and is first ARBs to get FDA approval. Losartan got its FDA approval in 1995. Angiotensin II receptor blocker (ARBs) are one of the most commonly used drugs for hypertension. They are often prescribed as first-line antihypertensive agents in patients with diabetes and renal disease.
- Losartan is a biphenylyltetrazole in which a 1,1’-biphenyl group is attached at the 5-position and has an additional trisubstituted imidazol-1-ylmethyl group at the 4′-position.
Indications of Losartan
- To treat hypertension in patients older than 6 years. Used alone or in combination with other anti-hypertensive drugs like hydrochlorothiazide, CCBs (calcium channel blocker) etc.
- To treat diabetic nephropathy with elevated serum creatinine and proteinuria in patients with hypertension and type 2 diabetes.
- Used to reduce risk of stroke in patients with hypertension and left ventricular hypertrophy.
- Some of its off label uses include Marfan syndrome, acute coronary syndrome, stable coronary artery disease.
Mechanism of action of Losartan
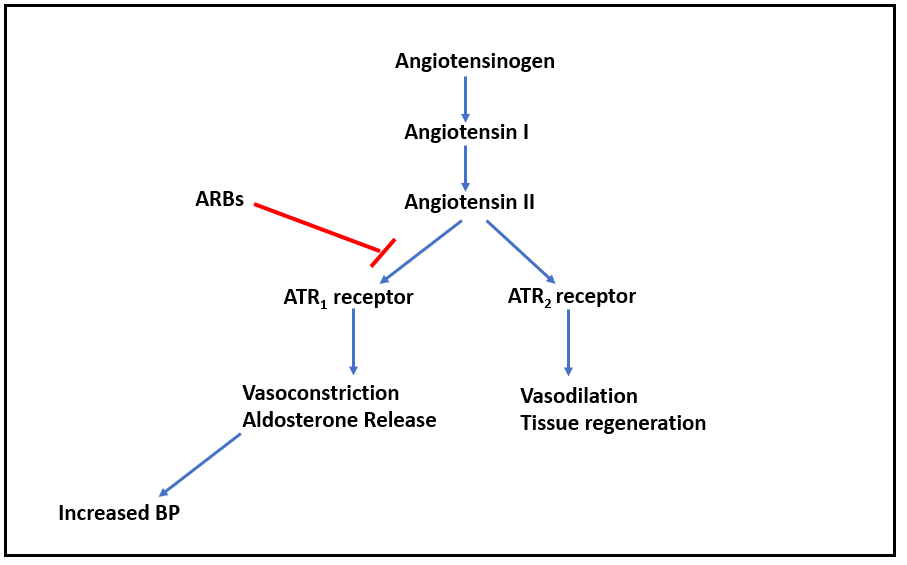
Figure- Mechanism of action of Losartan other ARBs
- Mechanism of action of losartan is like other ARBs; by competitively antagonizing angiotensin II type 1 (ATR1) receptor.
- Angiotensin II is a potent vasoconstrictor. It binds to two type of angiotensin II receptors (ATR): ATR1 and ATR2. The ATR1 receptors are abundant in the vessels, brain, heart, kidney, adrenal gland, and nerves while ATR2 are only available in small amounts in the adult kidney, adrenal gland, heart, brain, uterus, and ovary. Activation of ATR1 receptors cause contraction of vascular smooth muscle and increase in aldosterone resulting in increased in sodium reabsorption in the proximal tubule. This causes generalized vasoconstriction.
- Losartan blocks ATR1 receptor and prevents pressor effect of angiotensin II and prevents aldosterone synthesis and release which results in decreased sodium and water retention. It causes increase in urinary flow leading to increased excretion of sodium, calcium, magnesium, chloride, uric acid, and phosphate.
Pharmacokinetics of Losartan
- It is administered via oral route and reaches peak plasma level after 1-3 hours of oral administration. It undergoes metabolism in liver by CYP2C9 and CYP3A4. One of its metabolites, 5-carboxylic acid metabolite EXP 3174 is active and is more potent than losartan in blocking angiotensin At1 receptor. Plasma half-life of losartan is around 2.5 hours and of EXP 3174 is around 6 hours.
- Excretion takes place both by renal and hepatic route.
Adverse effects
- Common side effects include headache, dizziness, drowsiness, GI disturbances, rash, and fatigue. It can also cause first dose hypotension and orthostatic hypotension.
- Losartan may cause hyperkalemia in patients with renal insufficiency so potassium level should be continuously monitored. It can precipitate renal failure in patients with bilateral renal artery stenosis and with low, fixed renal blood flow.
- Chances of cough and angioedema are rare (less than ACE inhibitors).
Drug Interaction
- It’s use with aliskiren in diabetic patient is contraindicated due to risk of severe cardiovascular and renal side effects.
- Its use with ACE inhibitor is not recommended due to similar mechanism of action and similar adverse effects.
- As it has chances of causing hyperkalemia, concurrent use of potassium supplements and salt substitute containing potassium may cause excessive increase in potassium level.
Contraindications
- Contraindicated in patients hypersensitive to losartan or any of its compounds.
- When used in pregnancy, it can cause oligohydramnios. So, it contraindicated in pregnancy. Its use is not recommended during pregnancy.
- Should be used with caution in patients with history of or current hepatic or renal impairment.
- It is avoided in bilateral renal artery stenosis and used with caution in patient with renal artery stenosis.
References
- https://www.ncbi.nlm.nih.gov/books/NBK526065/
- https://go.drugbank.com/drugs/DB00678
- https://www.nature.com/articles/hr200842.pdf?origin=ppub
- Soffer BA etal. Effects of Losartan on a Background of Hydrochlorothiazide in Patients with Hypertension. 1995; 26 (1):112–117.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Pharmacology and pharmacotherapeutics. 24th edition.