- Tobramycin belongs to class of drug called aminoglycoside antibiotics. Tobramycin is derived from Streptomyces tenebrarius. It was approved by FDA in 1975. Tobramycin contains 4,6-disubstituted 2-deoxystreptamine (DOS) ring. It is also in the WHO’s List of Essential Medicines.
- The name ‘aminoglycoside’ is given as the structure consist of 2 amino sugars joined by glyosidic linkage to central hexose nucleus. Some other FDA approved aminoglycosides include streptomycin, gentamicin, amikacin etc.
Indications of tobramycin
- Systemic tobramycin is used to treat serious bacterial infection in pediatric and adult patients. Such bacterial infection includes lower respiratory tract infection, septicemia, intra- abdominal infections, serious infection of CNS like meningitis, skin, bone, and skin structure infections, recurrent and complicated urinary tract infections.
- Its ophthalmic form is used to treat external ocular infection in children and adults.
- Inhaled tobramycin is used in management of cystic fibrosis due to Pseudomonas aeruginosa in patients of age more than six years. However, its use is not recommended in patients with FEV (Forced Expiratory Volume) of <25% and >80% and in patients with Burkholderia cepacia.
Anti-bacterial spectrum
- It has activity against various gram-negative and some gram-positive bacteria. Tobramycin is active against gram negative organisms like E. coli, Klebsiella pneumoniae, Acinetobacter spp., Neisseria spp., Pseudomonas aeruginosa, Haemophilus spp., Moraxella lacunata etc. It is more active than gentamicin against P. aeruginosa (2 to 4 times).
- Also active against some gram-positive bacteria like Staphylococcus spp., including vancomycin resistant and methicillin resistant strains, Mycobacterium spp. And Streptococcus spp.
Resistance
- Resistance to tobramycin may occur due to modification of target site and reversible down regulation of drug uptake inside bacteria. Enzymes that can modify target site and develop resistance include aminoglycoside acetyltransferase (AACs), nucleotidyltransferases (ANTs) and phosphotransferases (APHs).
Mechanism of action of tobramycin
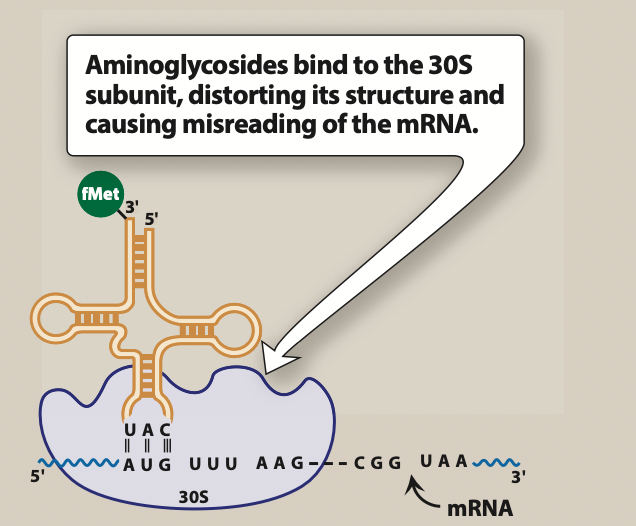
Figure 1- Mechanism of action of tobramycin and other aminoglycosides (Source- Lippincott’s Illustrated Reviews)
- Tobramycin had similar mechanism of action as of other aminoglycoside antibiotics. It diffuses through porin channels of gram-negative bacteria and enter periplasmic space. Once inside the cell, it binds to 16S ribosomal RNA of 30S ribosomal subunit and interfere with protein synthesis initiation, block translation of m-RNA and prematurely terminate the synthesis of protein. This results in production of toxic or non-functional proteins and disruption of normal protein synthesis.
Pharmacokinetics of tobramycin
- It can be administered via systemic (IM or IV), topical, ophthalmic and inhalation route. Administration via oral route may lead to poor absorption due to poor lipid solubility. Its absorption is rapid after IM administration.
- When it is used by IV route, it needs to be diluted with 0.9% sodium chloride injection or 5% dextrose injection. It is infused over 20 to 60 minutes and should be administered separately without premixing with other drugs. Even while using via nebulizer for inhalation, it should not be mixed with other drugs or dornase alfa.
- Binding to plasma proteins is less (almost negligible). Excretion takes place via renal route.
Adverse effects
- Some of its serious side effects include ototoxicity, nephrotoxicity, and neurotoxicity similar to other aminoglycosides. Risk of these effects may increase during long term therapy with tobramycin. Ototoxicity occurs due to loss of hair cells in inner ear and is irreversible. Nephrotoxicity is reversible and is due to binding of tobramycin to renal cortical tissue and proximal tubular necrosis. Neurotoxic effect is due to interference of acetylcholine release. It can also cause drug induced myasthenic syndrome which usually ceases if medication is stopped.
- Some reports have suggested association of Clostridium difficile associated diarrhea (CDAD) with tobramycin injection which may be mild to fatal. If CDAD is suspected, tobramycin treatment should be stopped.
Drug Interaction
- Concurrent use with other neurotoxic and nephrotoxic agents like other aminoglycosides, cisplatin, vancomycin, colistin, polymyxin B may increase its risk of neurotoxic and nephrotoxic effects. So, this combination should be avoided.
- Some diuretics like furosemide and ethacrynic acid may increase its toxicity when used together. So, these drugs should be avoided while using tobramycin.
- It shows synergistic effects with cephalosporin and carbenicillin.
Contraindications and warning
- Tobramycin is pregnancy category D medicine. It can cross placenta and cause congenital deafness in child. So, it is not used in pregnant women.
- Contraindicated in patients hypersensitive to tobramycin and other aminoglycoside antibiotics.
- Patients with myasthenia gravis (MG) should not take tobramycin as it can trigger or deteriorate symptoms of MG.
- Should be used with caution in elderly patients, renal patients and patients with Parkinson’s disease.
References
- Brogden RN etal. Tobramycin: a review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs. 1976; 12(3): 166-200.
- Lode H. Tobramycin – A review of therapeutic uses and dosing schedule. Current Therapeutic Research. 1998; 59 (7): 420-453.
- https://go.drugbank.com/drugs/DB00684
- https://www.ncbi.nlm.nih.gov/books/NBK551695/
- https://www.sciencedirect.com/referencework/9780080552323/xpharm-the-comprehensive-pharmacology-reference
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- A Textbook of Clinical Pharmacology and Therapeutics.
- Goodman and Gillman’s Manual of Pharmacology.