- Entecavir is guanosine nucleoside analogue used as anti- hepatitis B drug. Entecavir got its FDA approval in March 2005. It is included in World Health Organization’s List of Essential Medicines. Entecavir belongs to class of nucleoside reverse transcriptase inhibitors.
- Hepatitis-B is a major worldwide problem. It is a life-threatening liver infection caused by hepatitis B virus. It is transmitted from infected person to non-infected person via different routes like sexual contact or mucosal surface contact (via saliva, semen, blood etc), from mother to newborn. Some other agents used for HBV infection include lamivudine, telbivudine, tenofovir, adefovir etc.
- Entecavir is available in market under brand names Baraclude.
Indications of entecavir
- Used in treatment of chronic hepatitis B infection.
- It is also active against lamivudine resistant strains of HBV (Hepatitis B virus).
Mechanism of action of entecavir
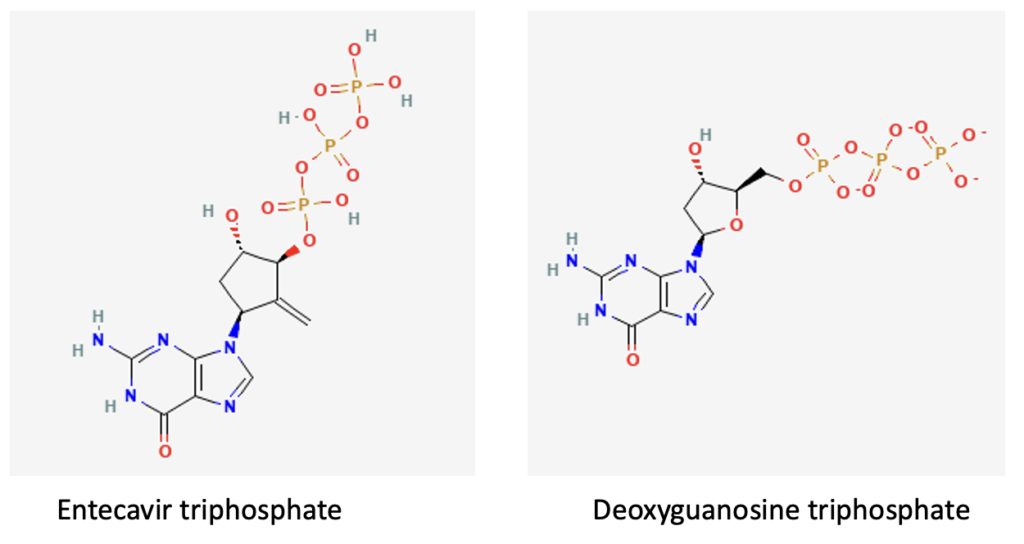
Figure- Entecavir triphosphate competes with deoxyguanosine triphosphate to enter viral DNA.
- Entecavir once administered, is activated to 5- triphosphate metabolite, which competes with natural substrate deoxyguanosine triphosphate to enter viral DNA. It than inhibits 3 major activities of reverse transcriptase viral RNA dependent HBV polymerase (reverse transcriptase) which are: base priming, reverse transcription of negative strand from pregenomic messenger RNA and synthesis of positive strand of HBV DNA. These all results in inhibition of HBV replication leading to decrease in number of HBV in blood.
- It is potent and highly selective for HBV.
Pharmacokinetics
- It is administered via oral route in the form of tablet or solution. Its bioavailability is 100% which may decrease when administered with food. Hence, patients are advised to take entecavir in empty stomach.
- Binding to plasma protein is around 13%. It is phosphorylated to active triphosphate form. It is not substrate for cytochrome P450 enzyme system and doesn’t inhibit or induce cytochrome P450 system.
- Unchanged form of entecavir is excreted via renal route. Dosage adjustment is required in patients with renal dysfunction.
Adverse effects
- Some of its common adverse effects include headache, upper respiratory tract infection, fatigue, dizziness, cough, and upper abdominal pain.
- It is found tumorigenic in animals.
Drug Interaction
- Chances of drug interaction is low as it is not substrate of cytochrome P450 enzyme system. Some drugs like probenecid may inhibit its tubular secretion and prolong its serum concentration.
References
- Matthews SJ. Entecavir for the treatment of chronic hepatitis B virus infection. Clin ther. 2006 Feb; 28(2): 184-203.
- https://www.frontiersin.org/articles/10.3389/fphar.2015.00304/full
- https://go.drugbank.com/drugs/DB00442
- https://www.ncbi.nlm.nih.gov/books/NBK555945/
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Pharmacology and pharmacotherapeutics. 24th edition.