- Angiotensin II receptor blocker (ARBs) are one of the most commonly used drugs for hypertension. They are often prescribed as first-line antihypertensive agents in patients with diabetes and renal disease.
- They are widely used in clinical practice due to their proven well-known effectiveness and tolerability. About 25 % of hypertensive patients worldwide use ARBs.
- The first ARB to be approved by FDA (Food and Drug Administration) was losartan in 1995. Some other examples of ARBs are:
Telmisartan
Valsartan
Candesartan
Eprosartan
Mechanism of Action of Angiotensin Receptor Blocker
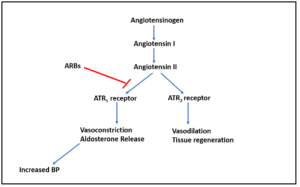
Figure 1- Mechanism of action of Angiotensin Receptor Blocker
- Inactive angiotensin I is converted to active angiotensin II by ACE (Angiotensin Converting Enzyme). Angiotensin II is a potent vasoconstrictor.
- It binds to two type of angiotensin II receptors (ATR): ATR1 and ATR2. The ATR1 receptors are abundant in the vessels, brain, heart, kidney, adrenal gland and nerves while ATR2 are only available in small amounts in the adult kidney, adrenal gland, heart, brain, uterus, and ovary.
- Activation of ATR1 receptors cause contraction of vascular smooth muscle and increase in aldosterone resulting in increased in sodium reabsorption in the proximal tubule. This causes generalized vasoconstriction.
- ARBs act by competitively antagonizing angiotensin II type 1 (ATR1) receptor. Thus, it prevents pressor effect of angiotensin II and prevents aldosterone synthesis and release which results in decreased sodium and water retention.
Pharmacokinetics of Angiotensin Receptor Blocker
- ARBs are well absorbed after oral administration.
- Most of them have long half-life to allow once a day dosing except valsartan which is twice a day.
- They are highly plasma bound and have large volume of distribution (except candesartan).
- Losartan undergoes first-pass metabolism and have active metabolites. All other ARBs have inactive metabolites.
- They are excreted through urine and feces.
| ARBs | Half-life (hours) | T max (hours) | Bioavailability |
| Losartan | 2 | 1-1.5 | 33% |
| Eprosartan | 5-9 | 1-3 | 63% |
| Irbesartan | 11-15 | 1.3-3 | 60-80% |
| Telmisartan | 24 | 0.5-1 | 43% |
| Valsartan | 6 | 2-4 | 23% (capsule)
50 % (solution) |
| Olmesartan medoxomil | 12-14 | 1.7-2.5 | 26% |
| Azilsartan medoxomil | 12 | 1.5-3 | 60% |
| Candesartan cilexetil | 9 | 2-5 | 42% |
Table 1- Pharmacokinetics of various ARBs (Source- Abraham et al, 2015)
Therapeutic Uses of Angiotensin Receptor Blocker
- Used as first line agent in treatment of hypertension. They are used alone or in combination with other antihypertensive drugs like diuretics.
- Used as substitute of ACE inhibitors in patients who cannot tolerate ACE inhibitors.
- They are used in hypertensive patients with diabetic nephropathy to slow the progression of nephropathy.
- Used in patients with heart failure or following myocardial infarction.
- Used to prevent recurrence of atrial fibrillation.
Adverse Effects
- They have excellent safety profiles alone and in combination with other anti-hypertensive drugs. They have better tolerability profile than ACE inhibitors.
- Common side effects include headache, dizziness, drowsiness, GI disturbances, rash and fatigue.
- It also causes first dose hypotension and orthostatic hypotension.
- In patients with renal insufficiency, it may cause hyperkalemia so potassium level should be continuously monitored.
- It can precipitate renal failure in patients with bilateral renal artery stenosis and with low, fixed renal blood flow.
- Cough and angioedema are rare (less than ACE inhibitors).
Contraindication
- When used in pregnancy, they may cause oligohydramnios which may result in even injury and death of fetus. Thus, it is contraindicated in pregnancy.
- They are contraindicated in patients with severe bilateral renal artery stenosis, aortic stenosis and coarctation of the aorta.
Drug Interaction
- They have few interactions with other drugs. They should not be combined with ACE inhibitors as it may increase risk of hypotension, hyperkalemia and renal impairment.
- As they increase blood potassium level, use of potassium supplements and salt substitute containing potassium may cause excessive blood potassium level.
- Rifampicin and fluconazole decrease the effect of losartan.
- Candesartan may interact with digoxin, warfarin and hydrochlorothiazide.
Comparison with ACE inhibitors
- They are pharmacologically different form ACE Inhibitors but have similar hypotensive efficacy.
- They have different mechanism of action. ACE inhibitors inhibit only one enzyme responsible for production of angiotensin II whereas ARBs cause complete blockade of angiotensin II activity.
- ARBs don’t affect bradykinin level as of ACE inhibitors so less chances of cough and angioedema than latter one.
- The choice between ARBs and ACE inhibitors depend upon familiarity and cost.
Use in COVID-19 infection
- The rapid spread of a novel coronavirus, SARS-CoV-2 has led to an ongoing pandemic of COVID-19. More than 7 million people worldwide are infected from COVID-19 as of June 2020.
- Angiotensin Converting Enzyme 2 (ACE 2) acts as a functional receptor for SARS-CoV-2 to enter host cells.
- Since ARBs upregulate ACE2 expression, serious concern arises regarding use of ARBs can increase mortality and morbidity of COVID-19.
- On other side, studies on animal also suggested a potential protective role of ARB in COVID-19 pneumonia. ARB prevented aggravation of acute lung injury in mice infected with SARS-CoV which is closely related to SARS-CoV-2.
- However, there is no appropriate evidence which support that ARBs either augment the susceptibility to SARS-CoV-2 or reduce the severity and outcomes of COVID-19 at present.
- It is recommended to continue ARBs in patients with cardiovascular disease and hypertension, especially those at high risk, according to guideline-directed medical therapy based on the currently available evidence. If COVID-19 infection occurs, individualized treatment decisions should be made based on patient’s hemodynamic status and clinical presentation.
References
- Khairnar AK, Baviskar DT, Jain DK. Angiotensin II receptor Blockers: An Overview. Int J Pharm Pharm Sci. 2012; 4(3):50-56.
- Kamal A, Fain C, Park A, Wang P, Leffler DA, Hutfless SM. Angiotensin II receptor blockers and gastrointestinal adverse events of resembling sprue-like enteropathy: a systematic review. Gastroenterology Report. 2019; 7(3): 162-167.
- Abraham HMA, White M, White WB. The Comparative Efficacy and Safety of the Angiotensin Receptor Blockers in the Management of Hypertension and Other Cardiovascular Diseases. Drug Saf. 2015; 38(1): 33–54.
- Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertension Research. 2020.
- Csaba AndrásDézsi. A review of clinical studies on angiotensin II receptor blockers and risk of cancer. International Journal of Cardiology. 2014; 177(3): 748-753.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.