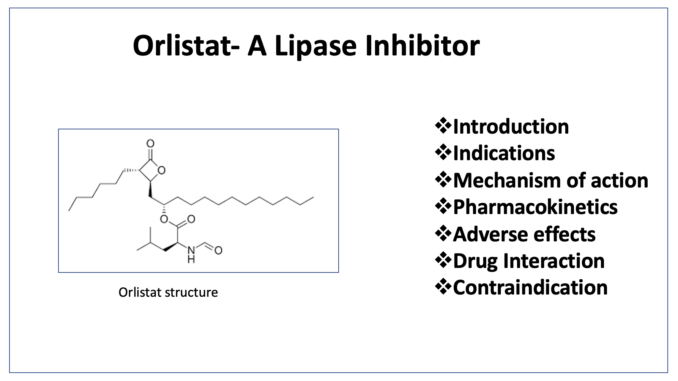
- Orlistat is lipase inhibitor used in treatment of obesity. Currently, orlistat is the only available agent of anti- obesity drugs of class lipase inhibitor. It is saturated derivative of endogenous lipstatin and is natural product derived from Streptomyces toxytricini. Orlistat is also known as tetrahydrolipstatin.
- It got its FDA approval in 1999 for use in obesity in combination with calorie reduced diet. Before 2012, orlistat was the only FDA approved medicine for long term use in weight loss. Nowadays, it is available both as prescription and OTC (over the counter) medication. It got its approval for OTC use in 2007.
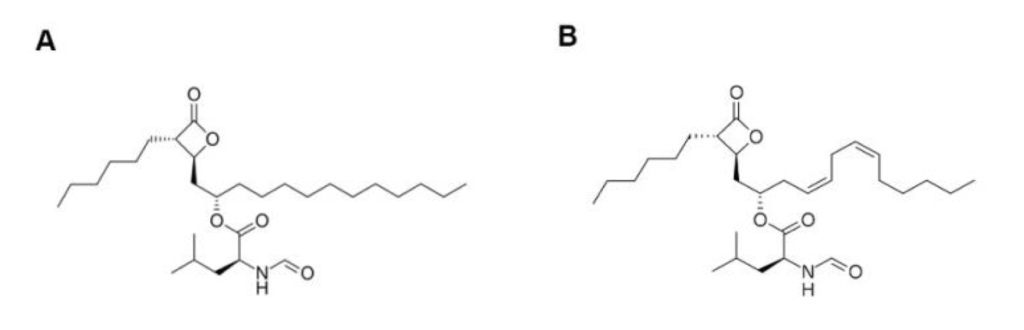
Figure- Chemical structure of orlistat (A) and lipstatin (B) (Source- Qi et al, 2018)
Indicator of orlistat
- Orlistat is FDA approved for management of obesity, both for weight loss and weight maintenance. It is used in:
- Obese patients with BMI (Body mass index) over 30 kg/m.
- Obese patients with BMI over 27 kg/m and with risk factors like diabetes, hypertension, and dyslipidemia.
- More effective when used in conjunction with diet and exercise. It helps in lowering BMI, waist circumference, body cholesterol level and LDL (low density lipoprotein) level.
Mechanism of action of orlistat
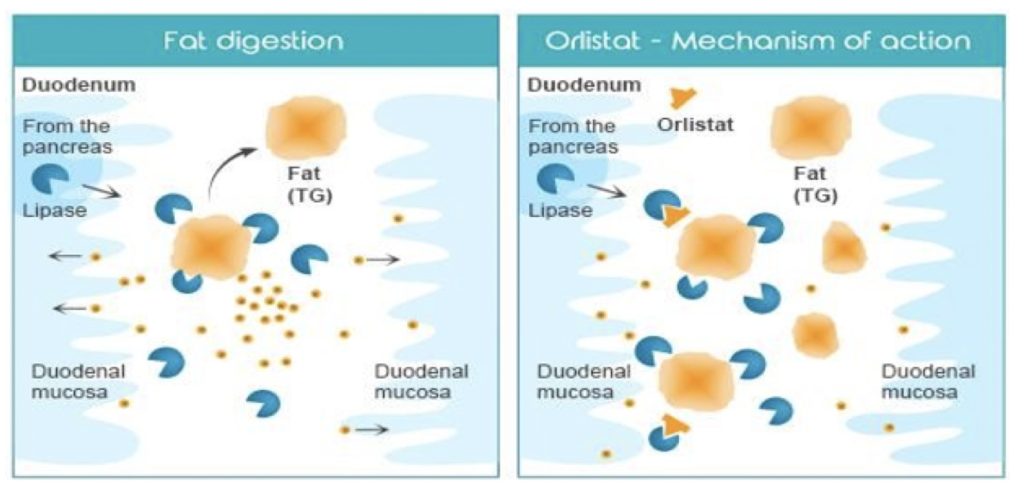
Figure Source- Chaudhary et al, 2020
- Orlistat acts as reversible, potent, and selective inhibitor of various lipase enzymes in gut. Lipase like gastric and pancreatic lipase play major role in digestion of fat by breaking triglycerides into free fatty acids and monoglycerides which are absorbable. Orlistat covalently binds to serine residues of active site of these lipases leading to their inactivation.
- Hence, there is no breakdown of fat into absorbable free fatty acids and monoglycerides which results in systemic absorption of dietary fat and loss of calories. Hence, the weight loss occurs. The undigested triglycerides than undergoes excretion via fecal route.
- It decreases fat absorption by around 30%. It has little to no activity against phospholipase, amylase, trypsin and chymotrypsin.
Pharmacokinetics of orlistat
- It is administered via oral route. It is available as 60 mg (for OTC use) and 120 mg (prescription use). Patients should take orlistat during or within 1 hour of having fat containing meal. If the meal doesn’t contain fat or if the patient doesn’t have meal, dose of orlistat which is to be taken with that meal can be omitted.
- It doesn’t undergo systemic absorption and systemic absorption is not necessary for its activity. It acts locally within GI tract. Its metabolism takes place within intestinal wall. Two major metabolites are M1, with a hydrolyzed 4-member lactone ring, and M3, M1 with the N-formal leucine moiety cleaved.
- Around 99% of administered drug binds to plasma proteins (like lipoproteins and albumin). It is excreted unchanged in feces.
Adverse effects
- Major adverse effects of orlistat are related to GI (gastrointestinal) system and are due to its lipase inhibition activity. Common GI effects include abdominal discomfort, liquid and soft stool, flatulence, fatty or oily stool, increased defecation, fecal incontinence, and oily rectal spotting. These effects occur within first week of starting orlistat therapy and decrease as the treatment continues. The risk of these effects increases if it is taken with meal containing high quantity of fat.
- It inhibits absorption of fat-soluble vitamins. Hence, patients who are on orlistat therapy should take multivitamin tablets containing vitamin A, D, E, K and beta-carotene every day.
- It increases risk of acute renal injury and renal stones. Unabsorbed fat due to lipase inhibition may bind with calcium and results in excessive oxalate which gets absorbed and deposited in kidney. This leads to oxalate nephropathy and increase in risk of renal stones.
- It is also associated with increased risk of cancer and osteoporosis.
Drug Interactions
- As it undergoes minimum systemic absorption, chances of significant drug-drug interaction are low.
- It can enhance bioavailability and pharmacological effect of pravastatin. It reduces absorption of lipophilic antiepileptics like valproate, gabapentin and vigabatrin. Orlistat also reduces absorption of other drugs like amiodarone, cyclosporine, and anti-retroviral agents. This is due to interference with intestinal absorption of these drugs by orlistat.
- When it is used in diabetic patients, weight loss in such patients may affect glycemic control. Hence, dosage of anti-diabetic agents needs to be adjusted.
Contraindications and warning
- It is contraindicated in patients hypersensitive to orlistat.
- Orlistat is pregnancy X drug. It is contraindicated in pregnant women and breastfeeding women.
- Not approved for children and adolescents under 16 years.
- It is contraindicated in patients with chronic malabsorption, cholestasis, anorexia, and bulimia.
References
- Heck AM etal. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacotherapy. 2000 Mar; 20(3): 270–279.
- Xiguang Qi. Review of the Clinical Effect of Orlistat. 2018 IOP Conf. Ser.: Mater. Sci. Eng. 30. 012063.
- https://go.drugbank.com/drugs/DB01083
- https://www.ncbi.nlm.nih.gov/books/NBK542202/
- https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323609623000072
- https://www.sciencedirect.com/referencework/9780444537164/meylers-side-effects-of-drugs
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Pharmacology and Pharmacotherapeutics. 24th edition.