- Dipeptidyl peptidase-4 inhibitors which are also known as gliptins are oral anti- hyperglycemic medications used in treating type 2 diabetes mellitus. Dipeptidyl peptidase-4 inhibitors are available in market for treatment of type 2 DM since 2006. Drugs belonging to class of dipeptidyl peptidase-4 inhibitors are:
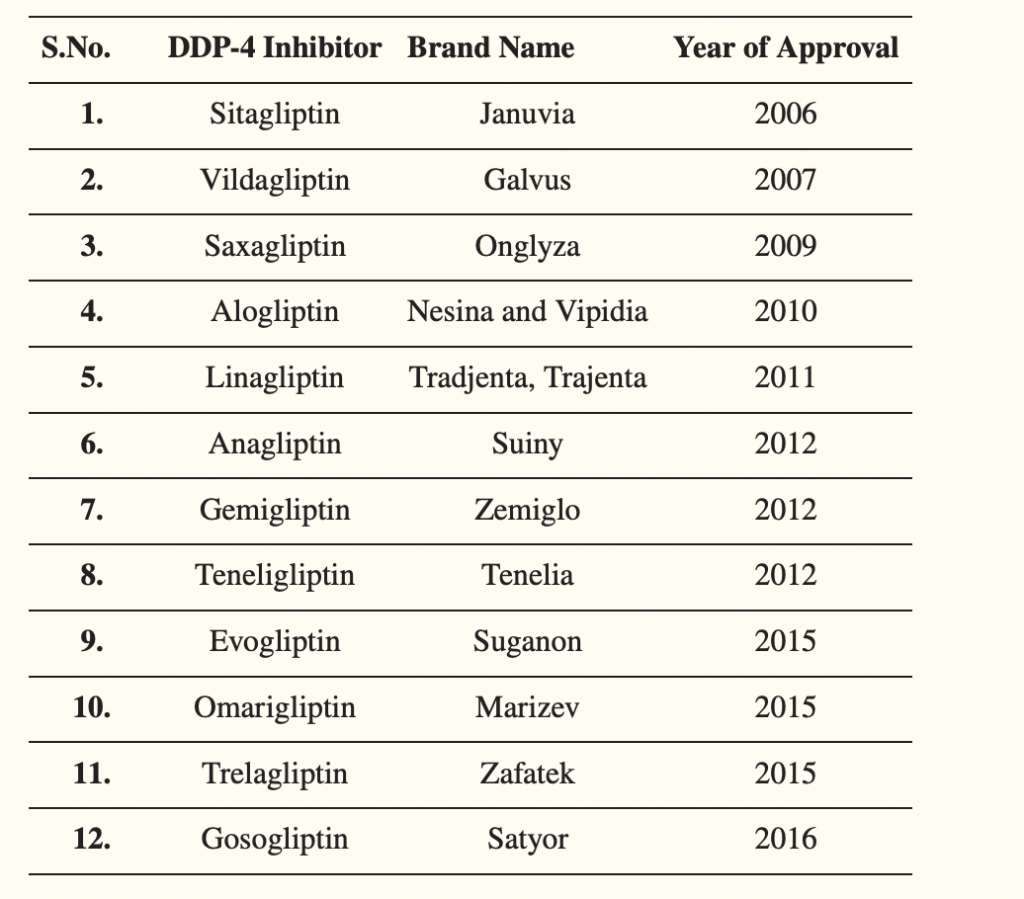
Table- List of commercially available Dipeptidyl peptidase-4 inhibitors (Source- Shaikh S et al. 2021)
Indications of Dipeptidyl peptidase-4 inhibitors
- Used in treating type 2 DM as monotherapy or in combination with other oral hypoglycemic agents like sulfonylurea, thiazolidinedione, metformin etc.,
- Used as alternative to sulfonylurea or pioglitazone in combination with metformin. Fixed dose combination of metformin and DPP-4 are commercially available.
- Can be used as monotherapy in type 2 DM patients who cannot tolerate metformin.
Mechanism of action of Dipeptidyl peptidase-4 inhibitors
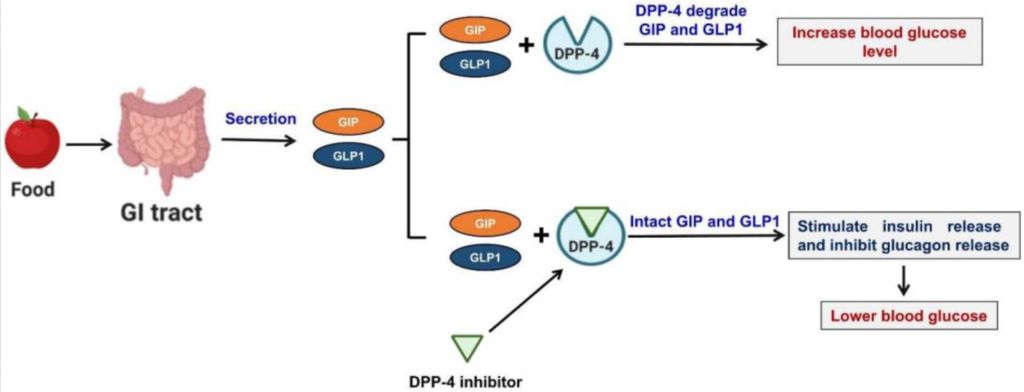
Figure- Mechanism of action of DPP-4 inhibitors (Source- Shaikh S et al. 2021)
- DPP-4 is a homodimeric type II transmembrane glycoprotein which is found on surface of different cell types. It acts as enzymes on incretin hormones, mainly GLP-1 (glucagon like peptide-1) and GIP (gastric inhibitory peptide). GLP-1 and GIP play major role in maintenance of glucose homeostasis.
- Both GLP-1 and GIP are released from GI (gastrointestinal tract) in response to nutrient consumption or increased blood glucose level. They augment glucose dependent insulin secretion, reduces glucagon secretion, decreases appetite, promote satiety, slows gastric emptying and cause weight loss. These incretins are released from GI tract within minutes of having food. DPP-4 enzyme causes immediate degradation of these incretins.
- DPP-4 inhibitors when used, inhibit DPP-4 enzyme, and inhibit degradation of GLP-1 and GIP. As a result, the level and half-life of GLP-1 and GIP increase which increases insulin secretion from pancreatic beta cells leading to decrease in fasting and post prandial hyperglycemia.
Pharmacokinetics of Dipeptidyl peptidase-4 inhibitors
- All dipeptidyl peptidase-4 inhibitors are administered via oral route and are well absorbed after oral administration. Presence of food doesn’t have any effect on their absorption. Their absorption occurs mainly in small intestine.
- Metabolic products of most of the DPP-4 inhibitors are not active. Exception is primary hydroxylated metabolite of saxagliptin, which is 2 fold less active than saxagliptin.
- Excretion of alogliptin, sitagliptin and saxagliptin takes place via renal route and excretion of linagliptin takes place via enterohepatic system. For dipeptidyl peptidase-4 inhibitors which are excreted via urine, dosage adjustment is required in renal patients.
Adverse effects
- They have good safety and tolerability profile.
- Common side effects include nasopharyngitis and skin lesions. They can also cause acute pancreatitis and severe joint pain. All DPP-4 inhibitors have label indicating the risk of acute pancreatitis. Some studies have found the increased risk of pancreatic cancer in patients using sitagliptin.
- They don’t have risk of hypoglycemia and weight gain like other oral antihyperglycemic agents.
Drug Interaction
- Drugs which inhibit CYP450 3A4/5 like clarithromycin, itraconazole, ritonavir may increase level of saxagliptin. So, dose of saxagliptin should be reduced when using with these agents.
Contraindications
- Contraindicated in type 1 diabetes and diabetic ketoacidosis.
- Contraindicated in patients who are hypersensitive to DPP-4 inhibitors.
- Should be used with caution in patients with history of pancreatitis.
References
- Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol., 19 June 2019.
- Zhong J etal. Recent Advances in Dipeptidyl-Peptidase-4 Inhibition Therapy: Lessons from the Bench and Clinical Trials. Journal of Diabetes Research. 2015; 606031: 1-14.
- Golightly LK et al. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012 Aug 1;51(8):501-14.
- https://www.ncbi.nlm.nih.gov/books/NBK542331/
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-dpp-4-inhibitors-type-2-diabetes-may-cause-severe-joint-pain
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.