
- Cyclophosphamide is most used alkylating agent. It is an alkylating agent with cytotoxic and immunosuppressive properties. It is chemotherapeutic agent used to treat different kinds of cancer.
- Cyclophosphamide falls under category of nitrogen mustard. It was developed by Norbort Brock and ASTA along with another related compound ifosfamide. FDA approved its clinical use as anti-cancer agent in 1959. It is included in World Health Organization’s List of Essential Medicines.
Indications of Cyclophosphamide
- It is important component of multiple drug regimen used to treat different kind of cancer including chronic lymphatic leukemia, Hodgkin and non-Hodgkin lymphoma, sarcoma and breast cancer.
- Used for treating various life-threatening autoimmune diseases like systemic lupus erythematous, microscopic polyangiitis, multiple sclerosis, Wegener’s granulomatosis and other autoimmune diseases.
Mechanism of action of Cyclophosphamide
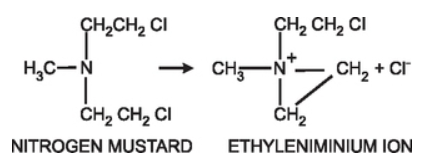
Figure 1- Formation of reactive ethyleniminium ion from nitrogen mustard (Source- Pharmacology and Pharmacotherapeutics, 24th edition)

Figure2- Mechanism of action of cyclophosphamide (Source- Lippincott’s Illustrated Reviews)
- It is a prodrug. After administration, it is biotransformed in liver by CYP 450 system to active hydroxylated intermediates – 4-hydroxyphosphamide. The hydroxylated intermediates are carried to tumor cells where they breakdown into active components phosphoramide mustard and acrolein. Phosphoramide mustard is responsible for antitumor effect and acrolein causes hemorrhagic cystitis.
- The reaction of phosphoramide mustard with DNA is cytotoxic step. Like other alkylating agents, it can transfer alkyl group to suitable receptor. Alkylating agents from highly reactive quaternary ammonium cation (ethyleniminium cation) which can alkylate groups like amino, phosphate and sulfhydryl. Hence, the resulting mustard alkylate DNA at guanine N-7 position and form DNA-DNA cross links. Cyclophosphamide can form both intra- and inter strand DNA cross links and DNA-protein cross links. It leads to inhibition of DNA synthesis and hence cause cell apoptosis.
- It is a non-phase specific cytotoxic agent.
Resistance
- Resistance to cyclophosphamide may occur due to decreased activation by CYP3A4 and CYP2B6, increased deactivation of agents, increased cellular thiol level, increased capacity to repair DNA, increased efflux or decreased entry of agents into tumor cells and decreased apoptotic response to DNA damage.
Pharmacokinetics of Cyclophosphamide

Figure 3- Activation of cyclophosphamide
- It is administered though oral or IV route. It is well absorbed orally. Metabolism takes place in liver and is metabolized to active hydroxyphosphamide and aldophosphamide (tautomeric form of hydroxyphosphamide) which are converted to inactive metabolites.
- It exhibits a low degree of plasma protein binding. Maximum plasma concentration is achieved after an hour of oral administration. Its half-life is around 7 hours. Cyclophosphamide and its metabolites are excreted in urine. About 14% of drug is excreted unchanged in urine. Its renal clearance depends on urine flow.
- It is a potent inducer of microsomal enzymes. It can induce its own metabolism after repeated administration.
Adverse effects
- It is mostly toxic to rapidly proliferating cells like hematopoietic system, hair follicles, gonads and epithelial cells of GI tract.
- The main toxic effects include alopecia, myelosuppression and hepatic damage. It can cause cystitis leading to fibrosis of the bladder. The cystitis is caused due to metabolite acrolein. This effect can be minimized by adequate hydration during cyclophosphamide therapy and administration of mesna- synthetic sulfhydryl compound. Mesna when given with cyclophosphamide and 4-8 hours later, react with toxic metabolite in urinary tract and neutralize them. The primary myelosuppression effect is leukopenia.
- It can cause nausea, vomiting and anorexia. Nausea and vomiting may be severe. High dose of cyclophosphamide is related to acute cardiac toxicity. Immunosuppressive property can cause infection like fever, chills, slow-wound healing.
- It possesses teratogenic effect and can cause birth defect if administered to pregnant women.
Drug-drug interaction
- Drugs like busulphan, allopurinol, chloramphenicol, ciprofloxacin and chlorpromazine inhibit its metabolism. Concurrent administration with drugs like dexamethasone, phenytoin, phenobarbitone and rifampicin induces its metabolism.
Contraindications
- Contraindicated in pregnancy due to its teratogenic effects. It is contraindicated in lactating mother.
- Contraindicated in patient with neutropenia, active infection or bladder infection.
References
- Zhang J, Tian Q, Zhou SF. Clinical Pharmacology of Cyclophosphamide and Ifosfamide. Current Drug Therapy. 2006; 1(1).
- Ahmed AR. Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol 1984; 11(6): 1115-26.
- Jonge ME, Huitema ADR, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clinical Pharmacokinetics. 44(11).
- Haubitz M et al. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Clinical Nephrology- Epidemiology- Clinical Trials. 2002; 61(4): 1495-1501.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- https://www.ncbi.nlm.nih.gov/books/NBK553087/