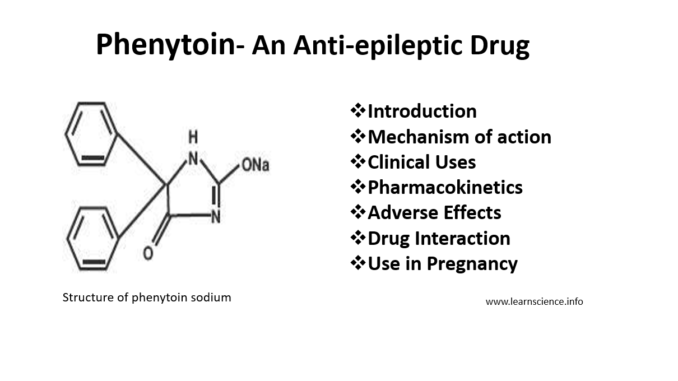
- Phenytoin which is also known as phenytoin sodium or diphenylhydantoin is commonly used anti-epileptic drug. It is classified under hydantoin derivatives and is structurally related to barbiturates.
- It was first synthesized by Heinrich Biltz in 1902 and it’s use to treat seizure without producing sedation effect was discovered by H. Houston Merritt and Tracy Putnam in 1938. It was approved by FDA in 1953 for use in seizures.
- From the time of its discovery till today, it has been investigated as treatment for more than 100 diseases.
Mechanism of action of Phenytoin
- It acts by slowing recovery of voltage dependent sodium channels which results in decreased permeability of Na+ ions (stabilizing effect) in all neuronal membranes.
- Reduction in neuronal Na+ concentration decreases post-tetanic potentiation (PTP) which is responsible for spread of seizure activity and inhibit spread of seizure discharge in the brain and shortens the duration of after discharge.
Clinical Uses of Phenytoin
- It is effective in treatment of tonic-clonic and partial seizure. It is not effective against absence seizure.
- To treat cardiac arrythmia.
- In status epilepticus.
- In some cases of trigeminal and related neuralgias. (Carbamazepine is preferred in such neuralgias.)
Pharmacokinetics
- It is available in rapid release and extended release oral form. Once daily dosing is possible only with extended release formulation. It is insoluble and crystallizes out in intramuscular injection site, so this route is not preferred. IV phenytoin is irritant to veins and tissues because of high pH.
- Intestinal absorption varies according to patient. There is 50-fold variation in steady state plasma concentrations of phenytoin in patients taking the same dose. It binds to serum proteins, mainly albumin.
- Phenytoin is extensively metabolized by liver and only 5% is excreted in unchanged form. It is metabolized mainly by parahydroxylation and metabolite formed is inactive. Phenytoin metabolism is saturable. It can inhibit or induce degradation of other drugs and certain substrates can inhibit phenytoin metabolism and increase its plasma concentration.
- At plasma concentration below 10 mcg/ml, (sub-therapeutic), elimination is exponential and the plasma t½ is about 24 hours. As it approaches the therapeutic concentration of 20 mcg/ml, its metabolism becomes saturated; it then exhibits dose dependent elimination and the plasma concentration rises disproportionately to the dose increment; the elimination t½ increases.
Adverse Effects
- It is well tolerated within the therapeutic range (10-20 mg/dl). At higher plasma level (˃20 mg/dl), the half life increases to 35 hours or longer and even a slight increase in dose can cause increased toxicity. The toxicity may disappear quickly if daily dose is reduced.
- Allergic effects like rashes, urticaria, drug fever may occur.
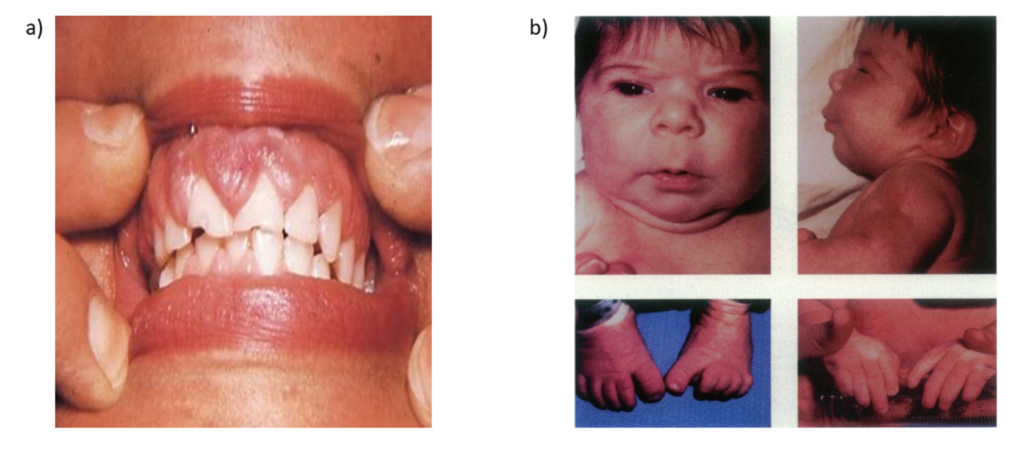
- Chronic administration is associated with gingival hyperplasia (hyperplasia of gums with bleeding). It may occur after the first month of treatment. The gums return to normal condition within one year after discontinuation of drug.
- Phenytoin facis may occur due to long term therapy. It is characterized by hypertrophy of facial sub-cutaneous tissue and coarsening of facial features.
- It can cause drowsiness, fatigue, confusion and headache. Large dose may cause a vestibulo-cerebellar syndrome characterized by vertigo, ataxia, nystagmus and dysarthria. Ocular pain with blurred vision, hallucinations and delusion are rare.
- Hydantoin when administered during first trimester of pregnancy, can cause fetal hydantoin syndrome. Children may develop wide mouth, short neck, mild webbing of the neck, hypoplastic nails, congenital heart defects and mental subnormality.
- Hypertrichosis in children, osteomalacia and hyperglycemia are rarely observed.
- Rapid IV administration may cause cardiovascular collapse and CNS depression.
Drug Interaction
- Isoniazid, cimetidine, cotrimoxazole and coumarin anticoagulants inhibit phenytoin metabolism, leading to its rise in plasma level and toxicity. Rifampicin and ethanol decrease plasma phenytoin level because of hepatic enzyme induction.
- Phenytoin competes with drugs such as sulfonamides, salicylates like aspirin and vitamin B 12 for binding to plasma proteins.
- It accelerates metabolism of drugs such as vitamin D, folate, glucocorticoids, gonadal steroids, thyroxine, doxycycline, warfarin and carbamazepine and reduce their therapeutic efficacy. Thus, it may cause osteomalacia and megaloblastic anemia.
Phenytoin in pregnancy
- A pregnant woman should not start taking phenytoin until suggested by her doctor. If a woman on phenytoin therapy becomes pregnant, she should not stop this medicine without consulting doctor.
- Phenytoin may cause harm to unborn baby but having seizure during pregnancy will harm both mother and baby. So, the benefits and adverse effects of phenytoin should be outweighed to continue in pregnancy.
References
- Karthikeyan M. Therapeutic Applications of Phenytoin. Asian Journal of Pharmaceutical and Clinical Research. 2009; 2(3):1-14.
- Fabiana CMH, Frederick SR, Xavier M. Side Effects of Phenytoin in the Oral Cavity: A Review. J of Oral Health Dent Sci. 2(1): 1-5.
- Hesselink JMK, Kopsky DJ. Phenytoin: 80 Years Young, From Epilepsy to Breast Cancer, a Remarkable Molecule with Multiple Modes of Action. J Neurol. 2017; 264(8): 1617-1621.
- Wu MF, Lim WH. Phenytoin: A Guide to Therapeutic Drug Monitoring. Proceedings of Singapore Healthcare. 2013; 22(3): 198-202.
- https://www.drugs.com/phenytoin.html
- Pharmacology and pharmacotherapeutics. 24th edition.
- A Textbook of Clinical Pharmacology and Therapeutics.
- Goodman and Gillman’s Manual of Pharmacology.
I like that briefing it’s concerning for health activity thanks!