- Olmesartan is drug belonging to class of ARBs (Angiotensin Receptor Blocker). Angiotensin II receptor blocker (ARBs) are one of the most used drugs for hypertension. They are often prescribed as first-line antihypertensive agents in patients with diabetes and renal disease.
- It was approved by FDA for medical use in 2002. It is also available as generic medicine nowadays. Other ARBs available in market includes losartan, telmisartan, valsartan, candesartan etc.
- Olmesartan is available as prodrug Olmesartan medoxomil which is converted in vivo into active form Olmesartan.
Indications of Olmesartan
- It is used alone or in combination with other anti-hypertensive drugs like diuretics and calcium channel blockers to treat hypertension.
- It is mostly used in patients who cannot tolerate ACE inhibitors.
- Used to treat Type 2 diabetes associated nephropathy to slow progression of nephropathy.
- Its off label uses include secondary prevention of myocardial infarction and in treatment of stable coronary artery disease.
Mechanism of action of Olmesartan
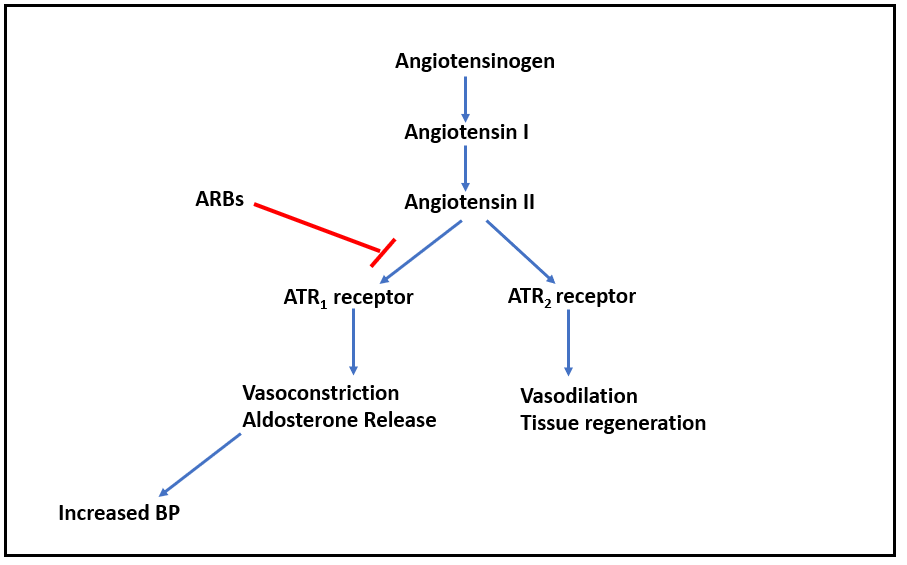
Figure- Mechanism of action of olmesartan and other ARBs
- It binds to angiotensin receptor 1 (AT1) and inhibits binding of angiotensin II to AT1. Angiotensin II is a potent vasoconstrictor and binds to two type of angiotensin II receptors (ATR): ATR1 and ATR2. The ATR1 receptors are abundant in the vessels, brain, heart, kidney, adrenal gland, and nerves while ATR2are only available in small amounts in the adult kidney, adrenal gland, heart, brain, uterus, and ovary.
- Activation of ATR1 receptors cause contraction of vascular smooth muscle and increase in aldosterone resulting in increased in sodium reabsorption in the proximal tubule. This causes generalized vasoconstriction.
- Inhibition of AT1 by Olmesartan prevents pressor effect of angiotensin II and prevents aldosterone synthesis and release which results in decreased sodium and water retention. The ultimate result is lowering of blood pressure.
- Blockade of AT1 receptor by Olmesartan also inhibits negative regulatory feedback within RAAS (Renin Angiotensin Aldosterone System) which plays major role in pathogenesis of heart failure, renal disease and cardiovascular disease.
Pharmacokinetics of Olmesartan
- It is administered via oral route. Prodrug Olmesartan medoxomil is deesterified to active form Olmesartan during GI absorption. Peak plasma level is achieved within 1.4-2.8 hours of oral administration. Its bioavailability is around 26% and is not affected by food intake.
- Olmesartan has high affinity for plasma proteins. Elimination takes place both via renal elimination and biliary excretion. Its half-life is around 10-15 hours.
Adverse effects of Olmesartan
- It has excellent safety profile when compared to other anti-hypertensive drugs.
- Some common side effects include headache, dizziness, upper respiratory infection, sinusitis, abdominal pain, diarrhea etc. Its long-term use may cause sprue enteropathy- like symptoms which include weight loss and osmotic diarrhea.
- Some of its rare but life-threatening side effects include angioedema, anaphylaxis, and severe renal failure. Other rare side effects include chest pain, dyspepsia, hyperkalemia, insomnia, hypotension and syncope.
Drug Interactions
- Its use with ACE inhibitors is contraindicated as this may increase risk of hypotension, hyperkalemia, and renal impairment. As it increases blood potassium level, co-administration of potassium supplements and salt substitute containing potassium may cause excessive blood potassium level.
- It’s use with aliskiren in diabetic patient is contraindicated due to risk of severe cardiovascular and renal side effects.
Contraindications
- It is contraindicated in patient hypersensitive to Olmesartan.
- Contraindicated in pregnancy (especially during second and third trimester).
- Contraindicated in patient with severe renal impairment, hyperkalemia, or previous history of hypotension.
- It is contraindicated in patient with biliary obstruction.
References
- https://www.statpearls.com/ArticleLibrary/viewarticle/706
- https://go.drugbank.com/salts/DBSALT001815
- https://en.wikipedia.org/wiki/Olmesartan
- Mire DE et al. A review of the structural and functional features of olmesartan medoxomil, an angiotensin receptor blocker. J Cardiovasc Pharmacol. 2005 Nov;46(5):585-93.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.