- Aliskiren is a first drug of the class direct renin inhibitors (DRI). It is only direct renin inhibitor approved for the treatment of hypertension. Hypertension is sustained elevation in blood pressure associated with increased risk of mortality and morbidity from cardiovascular and renal disease.
- It is developed by use of X-ray crystallography of the active site of renin and computational modelling. It was launched in 2007.
Use of aliskiren
- Aliskiren is used to treat primary (essential) hypertension alone or in combination with other agents. It is not recommended for initial treatment of hypertension.
- It can be used in hypertensive patients who cannot tolerate ARBs or ACEIs or with high plasma renin activity.
Mechanism of action of aliskiren
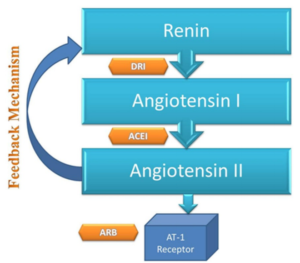
Figure- Action sites for ARBs, ACEIs and DRI (Direct Renin Inhibitor). (Source- Bhatti et al. 2015)
- RAAS (Renin Angiotensin Aldosterone System) play important role in long-term regulation of BP. Renin is a proteolytic enzyme produced and stored in kidney. It acts upon angiotensinogen to an inactive decapeptide ‘angiotensin I’ which is converted to active angiotensin I by angiotensin converting enzyme (ACE). Angiotensin II is potent vasoconstrictor and it also stimulate synthesis and release of aldosterone.
- Aliskiren is orally active, non- peptidic inhibitor of renin. It causes direct inhibition of plasma renin activity and reduce both angiotensin I and II level resulting in fall in BP.
- Although this seems similar to ARBs (Angiotensin Receptor Blocker) and ACEIs (ACE Inhibitors), they have unique mechanism to blunt plasma renin activity which is otherwise increased with ARB and ACEIs.
Pharmacokinetics of aliskiren
- It has limited systemic availability. Less than 3 % of parent compound is absorbed. Its half-life is around 24 hours which allows once daily dosing. It binds with plasma proteins (around 49 to 95%).
- Aliskiren doesn’t undergo hepatic metabolism so no significant effect of mild to severe hepatic impairment on single dose pharmacokinetics of aliskiren. It doesn’t influence cytochrome P450 enzymes so has little potential for drug- drug interaction. It is mainly excreted through feces. About 1% is excreted in urine.
Adverse effects
- It produces similar side effects as of ARBs. It can cause severe angioedema or subcutaneous swelling, increase in potassium level. The most common adverse effect is mild diarrhea which usually resolves within few weeks of treatment.
- It may cause headache, cough and gastroesophageal reflux.
Drug Interaction
- It reduces blood level of furosemide. Patients receiving combination of furosemide and aliskiren should be monitored closely for furosemide level. Concurrent administration with potassium sparing diuretics or potassium supplements will increase risk of hyperkalemia.
- Atorvastatin and ketoconazole increase aliskiren concentration and irbesartan decrease aliskiren concentration.
- It should be used with caution with ketoconazole and other P-glycoprotein inhibitors.
Contraindication
- Contraindicated in pregnancy and breastfeeding.
- Combination use of aliskiren with ARBs and ACEIs in diabetic patient is contraindicated because of risk of renal impairment, hyperkalemia and hypotension. A warning to avoid use of aliskiren with ARBs and ACEIs in patient with moderate to severe renal impairment was given by FDA.
References
- Brown MJ. Aliskiren. Circulation. 2008; 118: 773–784.
- “FDA Drug Safety Communication: New Warning and Contraindication for blood pressure medicines containing aliskiren (Tekturna)”. U.S. Food and Drug Administration(FDA). 19 January 2016. Retrieved 12 February 2020.
- Stachowiak IZ. Aliskiren – an alternative to angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in the therapy of arterial hypertension. Arch Med Sci. 2014 Aug 29; 10(4): 830–836.
- Meyler’s Side Effects of Drugs. The International Encyclopedia of Adverse Drug Reactions and Interactions. 16th edition.
- Bhatti A, Gazali Z. Can Aliskiren be Considered as a New Novel Drug for Hypertension? Cureus. 7(11): e375.