- Ramipril belongs to the class of drugs called ACE inhibitor (Angiotensin Converting Enzyme Inhibitor). It is second generation ACE inhibitor. Other ACE inhibitor available in market includes captopril, enalapril, fosinopril, quinapril and lisinopril.
- Ramipril was approved by FDA for medical use in 1989. It is also available as generic medicine.
Indications of ramipril
- For treating mild to severe hypertension.
- To manage symptomatic heart failure with reduced ejection fraction.
- It is used after MI (myocardial infarction) to prevent progression of asymptomatic heart failure with reduced ejection fraction.
- To reduce risk of stroke, MI, and death in old patients with major adverse cardiac events and high risk of atherosclerosis.
- Prescribed to slow progression of renal disease in patients with hypertension, diabetes, and microalbuminuria.
Mechanism of action of ramipril
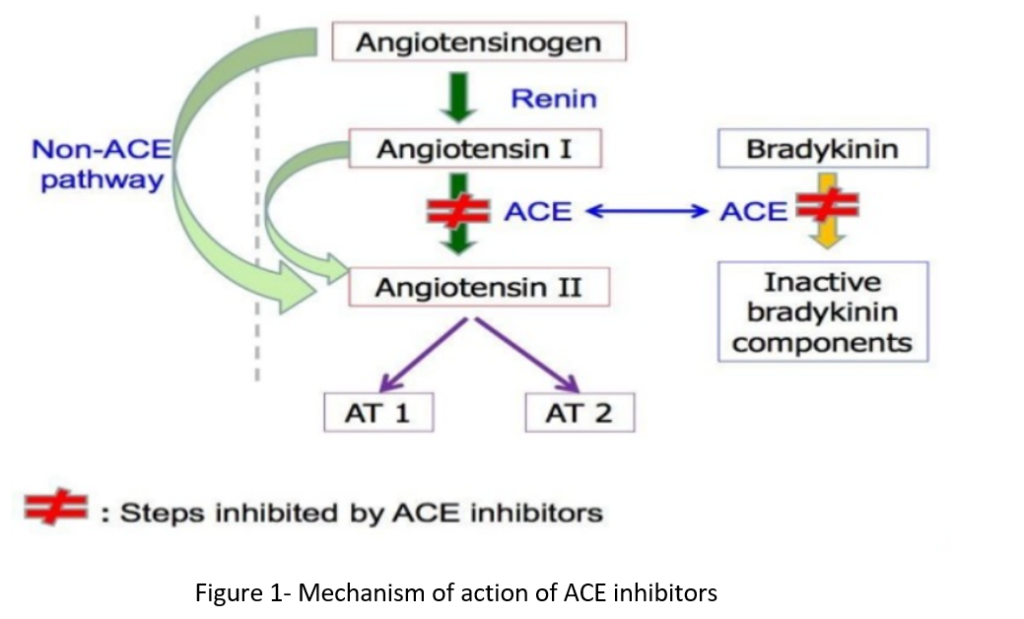
- Ramipril is prodrug and is converted to ramiprilat in liver and kidney. Ramiprilat is active and exerts its action by antagonizing the effect of RAAS system (Renin Angiotensin Aldosterone System). RAAS system play important role in regulation of hemodynamics, water, and electrolyte balance.
- It inhibits RAAS by inhibiting the enzyme, ACE (angiotensin converting enzyme). ACE cleaves inactive angiotensin I to active angiotensin II which is a potent vasoconstrictor. ACE is also involved in breakdown of bradykinin, a peptide that increases production of nitric oxide and prostacyclin, both of which are potent vasodilators.
- Ramiprilat competitively inhibit ACE and inhibit conversion of angiotensin I to angiotensin II. It results in prevention of:
- Pressor effect of angiotensin II.
- Stimulation of aldosterone synthesis and release resulting in decreased sodium and water retention.
- Metabolism of bradykinin by ACE.
- Thus, vasodilation of both arterioles and veins occurs because of decreased vasoconstriction (due to decreased angiotensin II) and enhanced vasodilation (due to increased bradykinin). The overall result is decrease of blood pressure.
Pharmacokinetics of ramipril
- It is administered via oral route and is absorbed rapidly. Peak plasma concentration of ramipril is achieved within one hour of administration and of ramiprilat within 3 hours. Administration with food decreases the rate of absorption but don’t affect the extent of absorption.
- Protein binding capacity of ramipril is 73% and of ramiprilat is around 56%. It is metabolized in liver where hepatic esterase enzyme cleaves ester moiety of ramipril and convert it to ramiprilat. It is also metabolized to some other inactive metabolites including glucuronides of ramipril and ramiprilat, diketopiperazine ester and acid.
- Excretion takes place mainly via kidney in the form of urine (around 60%). Around 40% of drugs and metabolites is excreted via feces.
- Ramiprilat’s plasma concentration decline in triphasic way. Initial half-life of 2-4 hours indicate extensive distribution in all tissues, intermediate half-life of 9-18 hours indicate clearance of free ramiprilat from plasma and terminal half-life of >50 hours indicate dissociation of ramiprilat from tissue ACE.
Adverse effects
- Most common adverse effect is persistent dry cough which occur in 5-30 % of cases. It is due to increased bradykinin in lung tissue. It is often mild but may be troublesome. Other common adverse effects include rash, fever, muscle cramp, GI disturbances and altered taste. It can also cause transient increase in serum creatinine level.
- First dose hypotension or postural hypotension may occur in people with severe hypertension who are on multidrug therapy including diuretics. Patient may feel dizziness and lightheadedness when suddenly stand up from sitting or lying position or may fall down which can cause bone fractures or head injuries. In such cases, it should be started in very small dose and preferably after stopping diuretics.
- Hyperkalemia may occur (particularly in patients with renal insufficiency) in 1-10% of patients so potassium level should be continuously monitored.
- Angioedema is rare but potentially life-threatening condition. It may involve head and neck or the intestine.
- Functional renal failure in patients with severe bilateral renal artery stenosis.
Drug Interaction
- Concurrent administration with drugs like aliskiren can increase risk of hypotension, nephrotoxicity, and hyperkalemia. Hence, it is not used with aliskiren.
- Concurrent administration of drugs like reteplase, temsirolimus and tenecteplase can increase risk of angioedema. Similarly, risk of other adverse effects may increase when ramipril is co- administered with drugs like sulindac, sulfasalazine, reviparin etc.
- Potassium containing food products can increase risk of hyperkalemia. So, patients are advised to avoid potassium containing food with ramipril.
Contraindication
- Contraindicated in patient hypersensitive to ramipril or other ACEIs.
- Contraindicated in patient with previous history of angioedema due to other ACE inhibitors.
- It is contraindicated in patient with hyperkalemia or hyponatremia.
- It is absolutely contraindicated in pregnancy due to risk of fetal malformation.
References
- https://go.drugbank.com/drugs/DB00178
- https://www.ncbi.nlm.nih.gov/books/NBK537119/
- https://www.medicinenet.com/ramipril/article.htm#what_is_ramipril_and_how_does_it_work_mechanism_of_action
- Frampton JE et al. Ramipril. An updated review of its therapeutic use in essential hypertension and heart failure. Drugs 1995 Mar; 49(3): 440-66.
- Douros A et al. Ramipril-Induced Liver Injury: Case Report and Review of the Literature. American Journal of Hypertension. 2013; 26(9): 1070–1075.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Pharmacology and pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.