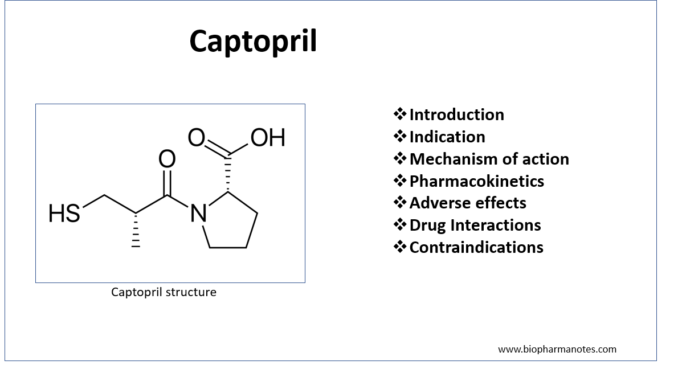
- Captopril is first ACE (Angiotensin Converting Enzyme) inhibitor discovered in 1981. ACE inhibitors are commonly used medicine in cardiovascular field.
Indications of captopril
- Used to treat hypertension. It is used alone or in combination with other agents.
- To treat diabetic nephropathy as it slows its progression and decreases albuminuria. Diabetic nephropathy is one of the leading causes of end stage kidney disease (ESKD).
- Used in left ventricular dysfunction after myocardial infarction. It can be used as additional agent to conventional therapy of severe CHF (Congestive Heart Failure) to reduce mortality and improve symptoms.
- Off label uses include in acute hypertensive crisis and Raynaud’s phenomenon.
Mechanism of action of captopril

Figure- Mechanism of action of ACE inhibitors (Captopril).
- Its benefit is due to suppression of RAAS (Renin Angiotensin Aldosterone System). ACE (angiotensin converting enzyme) cleaves inactive angiotensin I to active angiotensin II which is a potent vasoconstrictor. ACE is also involved in breakdown of bradykinin, a peptide that increases production of nitric oxide and prostacyclin, both of which are potent vasodilators.
- Mechanism of captopril is similar to other ACE inhibitors. It competitively inhibits ACE and hence inhibit conversion of angiotensin I to angiotensin II. It results in prevention of:
- Pressor effect of angiotensin II.
- Stimulation of aldosterone synthesis and release resulting in decreased sodium and water retention.
- Metabolism of bradykinin by ACE.
- Thus, vasodilation of both arterioles and veins occur as a result of decreased vasoconstriction (due to decreased angiotensin II) and enhanced vasodilation (due to increased bradykinin).
Pharmacokinetics of captopril
- It is orally effective agent and is absorbed rapidly after oral administration. It has bioavailability of around 75%. As its bioavailability is reduced by food by 25-30%, it is usually administered one hour before food.
- It is excreted through renal route in form of urine. About 40-50% is excreted as captopril and remaining are excreted as captopril disulfide dimers and captopril cysteine disulfide. Dosage adjustment is needed in patients with renal impairment.
- Its half-life is around 2 hours. The half-life may be longer in patients with CHF.
Adverse effects of captopril
- Most common and troublesome adverse effect is persistent dry cough which occur in 5-30 % of cases. It is due to increased bradykinin. It is often mild but may be troublesome. Other common adverse effects include rash, fever, muscle cramp, GI disturbances and altered taste (dysgeusia), tachycardia, chest pain, palpitations etc.
- First dose hypotension may occur in people with severe hypertension who are on multidrug therapy including diuretics. In such cases, it should be started in very small dose and preferably after stopping diuretics. Around 50% of patients experience acute fall in arterial pressure by 20%.
- It can cause proteinuria which gets cleared within six months even when captopril therapy is continued longer.
- Hyperkalemia may occur (particularly in patients with renal insufficiency) so potassium level should be continuously monitored.
- Angioedema is rare but potentially life-threatening condition.
- Functional renal failure in patients with severe bilateral renal artery stenosis.
Drug Interactions of captopril
- Concurrent use of potassium sparing diuretics or potassium supplements may increase risk of hyperkalemia.
- It may interact with aliskiren, lithium, sacubitril and drugs that weaken immune system like sirolimus. Concurrent administration with aliskiren may increase risk of hypotension, hyperkalemia, and nephrotoxicity. Risk or severity of angioedema is increased when captopril is combined with sacubitril. Captopril increases serum concentration of lithium and hence increases side effects associated with lithium.
- Drugs like acyclovir, adefovir may decrease excretion of captopril.
Contraindications
- Contraindicated in patients with known hypersensitivity to captopril or other ACE inhibitors.
- It is pregnancy category D drug. Hence, its use is discontinued as soon as pregnancy is confirmed.
- It is not co- administered with aliskiren in patients with diabetes mellitus.
- Not co- administered with or within 36 hours of administration of neprilysin inhibitor (sacubitiril).
- Contraindicated in patients with bilateral renal artery stenosis.
References
- Romankiewicz et al. Captopril: An Update Review of its Pharmacological Properties and Therapeutic Efficacy in Congestive Heart Failure. Drugs volume. 1983; 25: 6–40.
- Pediatric Cardiology. The Requisites in Pediatrics. 2006, Pages 237-275.
- https://www.webmd.com/drugs/2/drug-964/captopril-oral/details
- Captopril: Uses, Interactions, Mechanism of Action | DrugBank Online
- https://www.ncbi.nlm.nih.gov/books/NBK535386/
- A textbook of clinical pharmacology and therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Pharmacology and pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.