• Sulfonylurea are group of medicines used in treatment of type 2 diabetes. Sulfonylurea were discovered by chemist Marcel Jabon and coworkers in 1942, when they were studying sulfonamide antibiotics. They were the first type of diabetic pill to enter the market.
• Chemically, they are related to sulfonamide. They belongs to class of oral anti-diabetic agents.
• Type 2 diabetes is characterized by decreased insulin production from pancreatic beta cells and decreased tissue response to normal insulin (insulin) resistance. Type 2 diabetes accounts for 90-95 % of total case of diabetes worldwide.
• All sulfonylureas have similar mechanism of action and pharmacological actions but differ in pharmacokinetic properties.
Classification of sulfonylurea
They are classified into 1st, 2nd and 3rd generation based on their discovery.
1st generation sulfonylurea– Examples are tolbutamide, chlorpropamide, tolazamide and acetohexamide.
2nd generation sulfonylurea– Examples are glibenclamide, glipizide, gliclazide and gliquidone.
3rd generation sulfonylurea– Glimepiride is example. Sometimes, it is considered as second-generation agent.
• Second generation agents are significantly more potent than first generation agents. However, there is no clear evidence of superiority of second-generation agents in term of therapeutic efficacy.
Mechanism of action of sulfonylurea
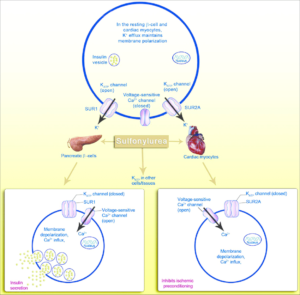
Figure 1- mechanism of action of sulfonylurea on pancreatic beta cells and cardiac myocytes (Source- Kalra et al, 2015)
In pancreatic beta cells
• They are insulin secretagogues and main mechanism involved is stimulation of insulin release from pancreatic beta cells.
• They bind to sulfonylurea receptors (SUR) linked to ATP dependent K channel (KATP) present in islet beta cells. Activation of receptors lead to closing of K channels causing depolarization which results in calcium influx into the cell with the release of stored insulin.
• They cause release of insulin slowly and for prolonged period.
In liver, muscle and adipose tissue
• They also increase sensitivity of liver, muscle and adipose tissues to insulin. And reduce hepatic glucose production and increase muscle uptake.
Pharmacological action of sulfonylurea
• Sulfonylurea lower the blood sugar level in non-diabetic and selected diabetics.
• They normalize metabolic state of diabetic.
• They lower elevated FFA level before they lower blood sugar level.
• Promote weight gain.
Pharmacokinetics
• All sulfonylureas have similar spectra of activities; thus, their pharmacokinetic parameter is their distinctive feature.
• They are effectively absorbed from GI tract when taken in empty stomach. Food and hyperglycemia may reduce their absorption. They are absorbed within 1-2 hours and peak level is observed within 4-6 hours.
• Large amount of most sulfonylurea (90-99%) bind to plasma proteins, especially albumin. They are mainly metabolized in liver.
• Some sulfonylureas are long acting (chlorpropamide, glibenclamide) and some are short-acting (tolbutamide, gliquidone).
• They are excreted form urine and feces.
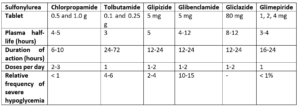
Table 1- Pharmacokinetic properties of different sulfonylureas (Source- Pharmacology and Pharmacotherapeutics. 24th edition)
Therapeutic Uses
• They are useful in treating symptomatic patients with type 2 diabetes who cannot achieve appropriate control with diet and exercise alone. They are often prescribed for diabetic patients who are not overweight or in whom metformin is contraindicated or metformin is not enough to obtain adequate glycemia control.
About 70% of patients show satisfactory response and remaining are termed as primary failures. Among the patients who show initial satisfactory response to sulfonylurea, 5-10% escape from control every year (secondary failure). It may be due to inadequate dose, poor compliance, progressive deterioration of pancreatic function, dietary excess and intercurrent disease.
Though they are second preferred drug to treat type 2 diabetes, they are still most used agents due to monodosing and their lower cost.
• In some cases of type 2 diabetes, they are used in combination with insulin.
• Chlorpropamide is used in diabetes insipidus.
Adverse Effects
• Chances of adverse effects are more common with first-generation sulfonylurea compared to second-generation sulfonylurea.
• The most common adverse effect is hypoglycemia. Hypoglycemia is precipitated by excessive dose, decreased food intake, presence of liver or kidney disease and vomiting. This is more common with long acting sulfonamides or when sulfonylurea is taken in combination with other anti-hyperglycemic agents.
• Weight gain is another common adverse effect associated with sulfonylurea.
• Allergic skin rashes may occur rarely. Other adverse effects are nausea, vomiting, cholestatic jaundice, aplastic and hemolytic anemia.
• Cardiovascular increased mortality and disulfiram like reaction are possible adverse effect when sulfonylurea is used for long term. Glyburide may act on SUR present on cardiac tissue and result in arrhythmia.
• Hyponatremia may occur due to potentiation of circulating ADH (anti-diuretic hormone) by sulfonylurea.
Drug Interaction
• They potentiate and prolong the CNS effect of barbiturates and other sedative-hypnotics.
• Drugs like oral anticoagulants, sulfonamide, phenylbutazone and salicylates can displace them from protein binding sites and potentiate their hypoglycemic effect. MAO inhibitors, warfarin, chloramphenicol and propranolol can decrease hepatic metabolism of sulfonylurea and produce hypoglycemic unresponsiveness. Alcohol can produce dangerous hypoglycemia when used by patients in sulfonylurea therapy.
• Drugs like rifampin can induce hepatic metabolism and decrease their concentration and efficacy.
• Concurrent administration of alcohol can produce disulfiram like reaction (flushing in susceptible individual). It is more common with chlorpropamide.
Contraindication
• They are contraindicated in type 1 diabetic patient.
• Contraindicated in pregnancy and lactating mother.
• Used with caution in renal and hepatic insufficiency.
References
- Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, featherstone TR. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. The Lancet Diabetes and Endocrinology. 2015; 3(1): 43-51.
- Khunti K, Chatterjee S, Gerstein HC, Zoungas S, Davies MJ. Do sulphonylureas still have a place in clinical practice? The Lancet Diabetes and Endocrinology. 2018; 6(10_: 821-832.
- Aquilante Sulfonylurea pharmacogenomics in Type 2 diabetes: the influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. 2010; 8(3): 359–372.
- Kalra S, Aamir AH, Raza A, Das AK, Khan AK, Shrestha D et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement. Indian Journal of Endocrinology and Metabolism. 2015; 19(5): 577-596.
- Furman BL. Sulfonylureas. xPharm: The Comprehensive Pharmacology Reference. 2007:1-2.
- Pharmacology and Pharmacotherapeutics. 24th
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th
- https://www.rxlist.com/sulfonylurea/definition.htm