- Voriconazole is synthetic, second- generation, triazole antifungal agent. Its structure is like fluconazole, but it has broad spectrum and expanded activity.
- It was approved by FDA under trade name Vfend in 2002. Voriconazole is included in World Health Organization’s List of Essential Medicines.
Indications of voriconazole
- It is used in treatment of invasive candidiasis. It is also active against fluconazole resistant candida species including C. glabrata and C. krusei.
- Used to treat serious infections caused by Fusarium and Scedosporium species.
- It is superior to and has replaced amphotericin B as drug of choice in treatment of invasive aspergillosis.
- Its off label uses include prophylactic treatment in neutropenic patients whose fever couldn’t be managed by other antibiotics and are at risk of developing fungal infection.
Mechanism of action of voriconazole
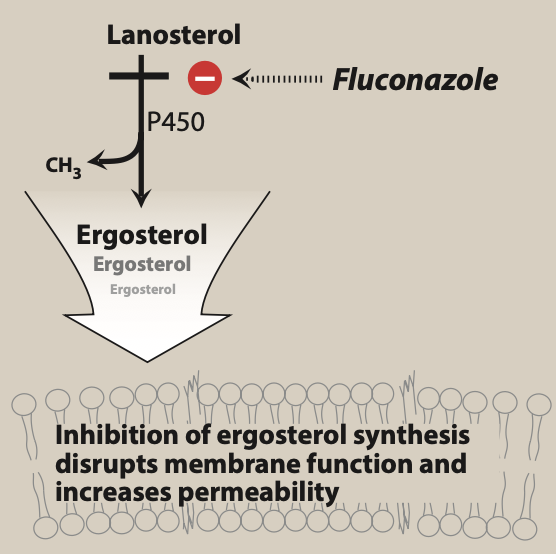
Figure- Mechanism of action of triazole antibiotics including voriconazole (Source- Lippincott’s Illustrated Reveiws Pharmacology, 6th edition)
- Its mechanism of action is similar to other azole antibiotics i.e., by inhibiting ergosterol synthesis. Ergosterol is major component of cell membrane of fungi.
- It binds to and inhibits fungal cytochrome P450 enzyme, C-14 a-demethylase which is involved in 14- alpha demethylation reaction of lanosterol to ergosterol. This inhibition in ergosterol synthesis cause impairment in membrane structure and function and results in inhibition of fungal cell growth.
Pharmacokinetics of voriconazole
- It is available for oral and IV administration. Oral bioavailability is nearly complete, around 96% in healthy adults. The bioavailability may be reduced in pediatric population and in transplant patients. Its bioavailability is reduced by high fat meal, so oral dose is administered 1 hour before or after food.
- It is distributed extensively in most of the tissues. Around 58% binds to plasma proteins. It undergoes extensive hepatic metabolism by cytochrome enzymes like CYP2C9, CYP3A4 and CYP2C19. The major metabolism is by CYP2C19 and major metabolite is voriconazole N- oxide.
- It is excreted via urine. Its half-life is around 6 hours. It exhibits non-linear kinetics. Higher doses may increase drug exposure disproportionately. Dosage reduction is required in patients with hepatic impairment.
Adverse effects
- It is generally well tolerated drug. However, it can cause hepatotoxicity so patients in voriconazole therapy should be monitored for hepatic function regularly. It can cause visual disturbances like blurred vision, photophobia, altered color perception after about 30 minutes of administration and may last for around more 30 minutes.
- It can cause rashes and prolongation of QTc interval. IV infusion of voriconazole may cause anaphylactoid reactions and transient visual or auditory hallucinations.
Drug Interactions
- As it undergoes extensive metabolism by enzymes like CYP2C19, CYP2C9 and CYP3A4, inducers or inhibitors of these enzymes may increase or decrease concentration of voriconazole.
- Drugs like rifampin or rifabutin may increase metabolism of voriconazole. Hence co-administration of voriconazole with these drugs is contraindicated. Similarly other drugs like efavirenz and NNRTIs (non- nucleoside reverse transcriptase inhibitors) also increase metabolism of voriconazole. Concurrent administration of voriconazole and omeprazole increase each other’s concentration. So, dose of omeprazole is reduced by half when starting voriconazole therapy.
- Voriconazole may inhibit metabolism and lead to accumulation of drugs like warfarin, phenytoin, sirolimus, tacrolimus and cyclosporine.
Contraindications
- It is pregnancy category D drug. It has teratogenic effects and hence is contraindicated in pregnancy.
- IV formulation is used with diluent sulphobutylether beta cyclodextrin sodium. This compound when accumulated has nephrotoxic and hepatotoxic effects. Hence, it should be avoided in patients with significant renal dysfunction.
- Contraindicated in patients with hypersensitivity to voriconazole.
- It is contraindicated with many drugs including rifampin, rifabutin, carbamazepine and herb St. John’s wort.
References
- A textbook of Clinical Pharmacology and therapeutics. 5th edition.
- Lippincott’s Illustrated Reviews Pharmacology. 6th edition
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- https://go.drugbank.com/drugs/DB00582
- Scott LJ et al. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs.2007; 67(2): 269-98.
- Thompsom GR et al. Pharmacology and clinical use of voriconazole. Expert Opinion on Drug Metabolism and Toxicology. 2010; 6(1): 83-94.