- Warfarin is commonly used anticoagulant used to prevent and treat blood clots that might result in heart attack, stroke and death.
- It is available both as generic and brand medicine. It was first used as rat poison in 1948. Warfarin was approved for medical use in United States in 1954. It is on the World Health Organization’s List of Essential Medicines.
- It belongs to the group of coumarin anticoagulants.
Mechanism of action of warfarin
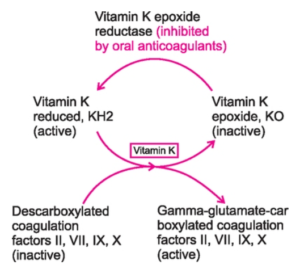
Figure 1- Vitamin K cycle and site of action of oral anticoagulants like warfarin
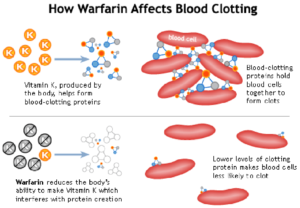
Figure 2- Action of warfarin on blood clotting
- Coagulation factors prothrombin, VII, IX and X which are synthesized in liver need to be in carboxylated form to be biologically active. These factors undergo vitamin-K dependent posttranslational modification, in which their glutamic acid residues are carboxylated to form ϒ-carboxyglutamic acid residues. Vitamin K undergoes oxidation to its epoxide form during this reaction.
- The epoxide form is converted back to its reduced form by vitamin K epoxide reductase enzyme. This helps in sustaining the carboxylation process.
- Warfarin inhibits vitamin K epoxide reductase and prevents regeneration of reduced form of vitamin K resulting in inhibition of carboxylation. Thus, vitamin K dependent synthesis of coagulation factors is inhibited.
- Warfarin treatment results in the production of clotting factors with diminished activity due to lack of ϒ-carboxyglutamyl side chains. Thus, it reduces blood clotting in patient.
Pharmacological actions of warfarin
Anticoagulant action
- Its anticoagulant action is not observed immediately. This is because they don’t destroy the circulating coagulation factors but prevent formation of active essential clotting factors.
- The anticoagulant effects can be overcome by the administration of vitamin K.
- Its anticoagulant action is measured by INR (International Normalized Ratio). In most of the cases, the goal of warfarin therapy is an INR of 2 or 3, whereas the goal may be 2.5- 3.5 for some mechanical valves.
Pharmacokinetics of warfarin
- It is rapidly absorbed after oral administration. The bioavailability is around 100% with little individual patient variation. It can also be administered through IV or rectal route. Food in GI tract can decrease its absorption. IM injection is not recommended because of risk of hematoma formation.
- It is highly bound to plasma protein albumin. This prevents its diffusion in cerebrospinal fluid (CSF), urine and breast milk. It can easily cross the placental barrier.
- Warfarin is a racemic mixture of R (weak) and S (potent) anticoagulant enantiomers. Both isomers are transformed into inactive metabolites by CYP450 system. The inactive metabolites are excreted in urine and feces.
- Half-life may range from 25-60 hours (average 40 hours). The duration of action is 2-5 days.
Therapeutic Uses
- Prevention and treatment of deep vein thrombosis and pulmonary embolism.
- For prevention of venous thromboembolism during orthopedic or gynecologic surgery.
- For stroke prevention in atrial fibrillation or prosthetic heart valves. Warfarin is the only anticoagulant approved by FDA in individual with mechanical heart valves.
- In acute myocardial infarction.
Adverse Effects
- It has narrow therapeutic index which makes difficult to maintain the patient within anticoagulation range.
- The main adverse effect is bleeding. It has a black box warning for bleeding risk. Minor bleeding can be controlled by drug withdrawal and administration of oral vitamin K. Severe bleeding may require greater doses of vitamin K administered through IV route.
- Skin lesions and necrosis may occur rarely. GI disturbances (nausea, vomiting, diarrhea),
- Purple toe syndrome- a rare, painful, blue tinged discoloration of toe is also observed. This is caused by cholesterol emboli released form atheromatous plaques.
- Administration during pregnancy causes birth defects and abortion.
Note- Daily INR (International Normalized Ratio) measurement and recognition of a rapidly increasing INR may be helpful in decreasing its adverse effects in hospitalized patients.
Drug Interactions
- It has numerous drug interactions that attenuate or potentiate its anti-coagulant effect.
- Concurrent use of drugs that affect bleeding such as NSAIDs (aspirin), selective serotonin reuptake inhibitor may increase risk of bleeding.
- Warfarin can be displaced from its protein binding sites by drugs like sulfonamide, chlorpropamide, phenylbutazone.
- Certain medication which increase the amount of warfarin (by inhibiting metabolism) and put at higher risk of bleeding includes amiodarone, isoniazid, metronidazole, sulfamethoxazole and voriconazole.
- Medications which stimulate CYP 450 and stimulate metabolism of warfarin may decrease the amount of warfarin. These medications include barbiturates, chronic alcohol ingestion, rifampin, carbamazepine and phenobarbital.
- It may interact with many herbs and spices.
Contraindications
- It is contraindicated in pregnancy due to its teratogenic effects. Heparin or low molecular weight heparin is used if anticoagulant therapy is needed during pregnancy.
- It is contraindicated in patients with active bleeding disease (gastric ulcer, wounds), or disease with increased risk of bleeding (liver disease, low platelet count).
- It is avoided in patients undergoing surgery.
- Avoided in people with protein C or protein S deficiency, as there is increased risk of skin necrosis in these thrombophilic conditions.
References
- Pirmohamed M. Warfarin: almost 60 years old and still causing problems. Br J Clin Pharmacol. 2006; 62(5): 509–511.
- Metersky ML, Eldridge N, Wang Y, Jaser L, Bona R, Eckenrode S etal. Predictors of warfarin‐associated adverse events in hospitalized patients: Opportunities to prevent patient harm. Hosp. Med. 2016; 11(4): 276-282.
- Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G et al. Association Between Use of Warfarin for Atrial Fibrillation and Outcomes Among Patients with End-Stage Renal DiseaseA Systematic Review and Meta-analysis. JAMA Netw Open. 2020; 3(4): e202175.
- Jain N, Reily RF. Clinical Pharmacology of Oral Anticoagulants in Patients with Kidney Disease. 2019; 14 (2): 278-287.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology. 6th edition.