- Kanamycin or kanamycin A belongs to class of drugs called aminoglycoside antibiotics. Aminoglycosides are important group of antibiotics which act against gram-negative bacteria. The name ‘aminoglycoside’ is given as the structure consist of 2 amino sugars joined by glyosidic linkage to central hexose nucleus. They are either derived from Streptomyces (ends in -mycin) or Micromonospora (ends in micin).
- Kanamycin is derived from Streptomyces kanamyceticus. Due to its toxicity and serious side effects, it was removed from World Health Organization’s List of Essential Medicines in 2019 and is rarely prescribed nowadays. Amikacin has replaced kanamycin in United States.
Indications of Kanamycin
Used for treating following infections caused my microorganism sensitive to kanamycin:
- Sepsis
- Meningitis
- Osteomyelitis
- Peritonitis
- Pneumonia
- Infected wounds
- Post-operational purulent complications
- Multi-drug resistant tuberculosis (MDR-TB)
Mechanism of action of Kanamycin
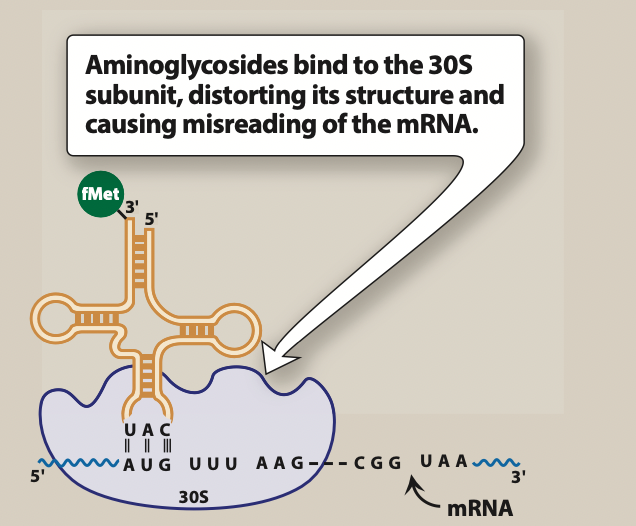
Figure- mechanism of action of kanamycin is like other aminoglycosides. (Source- Lippincott’s illustrated Reviews)
- Kanamycin and other aminoglycoside antibiotics diffuse through porin channels of gram-negative bacteria and enter periplasmic space. Once inside the cell, they bind to 30S ribosomal subunit and interfere with protein synthesis initiation, block translation of m-RNA and prematurely terminate the synthesis.
- Kanamycin specifically binds to 4 nucleotides of 16S rRNA (RNA component of 30S subunit of prokaryotic ribosome) and single amino acid of protein S12. This result in interference of initiation complex and misreading of mRNA which causes incorporation of incorrect amino acids into polypeptide complex. As a result, non-functional or toxic peptides are produced.
Anti-bacterial spectrum
- Used in treating infections caused by following pathogens:
- E. coli
- E. aerogenes
- K. pneumoniae
- S. marcescens
- Proteus species (both indole positive and indole negative).
Pharmacokinetics of Kanamycin
- It is available as oral, IV or IM form. However, absorption through oral or topical route is poor. It is rapidly absorbed after IM administration and peak plasma concentration reaches within an hour.
- Kanamycin along with amikacin and capreomycin are considered as same group as all three are administered by IV or IM route, excreted via renal route and have similar pharmacokinetic properties and adverse effects. Half-life of kanamycin is around 2.5 hours.
Adverse effects
- One of the serious side effects is cochlear damage. It can cause reversible impairment of vestibular function and can cause permanent hearing loss. Chances of developing ototoxicity is more in infants, elder people and in patients with impaired renal function.
- It has narrow therapeutic index like other aminoglycosides. Hypersensitivity reactions are common and can manifest as rashes, eosinophilia, and fever. Serious manifestations like blood dyscrasias, angioedema occur rarely. It can cause pain at site of injection.
- Nephrotoxicity is another common adverse effect which occurs due to accumulation and retention of streptomycin in proximal tubular cells. It can cause mild albuminuria or acute tubular necrosis. Chances of azotemia is rare.
- Acute neuromuscular blockade may occur due to rapid increase in concentration or concurrent administration with neuromuscular blockers. Chances of neuromuscular blockade is more when administered directly into peritoneum. However, this effect is less dangerous compared to that of streptomycin or neomycin. Patients with myasthenia gravis are more susceptible. It may be reversed with calcium gluconate infusion or by neostigmine.
- It can also cause optic neuritis and peripheral neuritis.
Drug Interactions
- Concurrent administration with loop diuretics or any other potent diuretics can potentiate its ototoxic and nephrotoxic effects.
- It enhances neuromuscular blockade property of non-depolarizing neuromuscular antagonists.
Contraindications
- Contraindicated in following conditions:
- Patients with myasthenia gravis.
- In patients who are allergic to kanamycin or any other aminoglycoside antibiotics including streptomycin, neomycin, amikacin etc.
- It is pregnancy category D drug. When administered in pregnancy, it can cross placenta and produce ototoxic effects.
- Used with caution in elderly patients and in patients with renal impairment.
References
- https://go.drugbank.com/drugs/DB01172
- https://en.wikipedia.org/wiki/Kanamycin_A
- https://www.rxlist.com/kantrex-drug.htm#description
- https://www.ncbi.nlm.nih.gov/books/NBK541105/
- https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/563437
- Meyler’s Side Effects of Drugs. The International encyclopedia of Adverse Drug reactions and Interactions. 15th edition.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- A textbook of clinical pharmacology and therapeutics.