- Ifosfamide is analog of cyclophosphamide. It is cytotoxic, antineoplastic agent and belongs to class of oxazaphosphorine alkylating agents.
- It is commonly used alkylating agent in cancer chemotherapeutics.
Indications of Ifosfamide
- It is used as single agent or in combination with other antineoplastic agents to treat different kind of cancer including:
- Germline tumor of testis
- Ovarian cancer
- Thymic cancer
- Osteosarcoma
- Non-hodgkin lymphoma
- Breast cancer
Mechanism of action of Ifosfamide
- It is a prodrug. After administration, it is bio transformed in liver by CYP 450 system to active hydroxylated intermediates. The hydroxylated intermediates are carried to tumor cells where they breakdown into active components phosphoramide mustard derivatives and acrolein. Phosphoramide mustard is responsible for antitumor effect and acrolein causes hemorrhagic cystitis.
- The reaction of phosphoramide mustard with DNA is cytotoxic step. Like other alkylating agents, it can transfer alkyl group to suitable receptor. Alkylating agents form highly reactive quaternary ammonium cation (ethyleniminium cation) which can alkylate groups like amino, phosphate and sulfhydryl. Hence, the resulting mustard alkylate DNA at guanine N-7 position and form DNA-DNA cross links. It can form both intra- and inter strand DNA cross links and DNA-protein cross links. It leads to inhibition of DNA synthesis and hence cause cell apoptosis. Active metabolites also cause upregulation of reactive oxygen species (ROS) which causes DNA damage and inhibit protein formation.
- It is a non-phase specific cytotoxic agent.
Resistance
- Resistance to ifosfamide may occur due to decreased activation by CYP3A4 and CYP2B6, increased deactivation of agents, increased cellular thiol level and rection of drug with thiols, increased capacity to repair DNA, increased efflux or decreased drug permeability and decreased apoptotic response to DNA damage.
Pharmacokinetics of ifosfamide
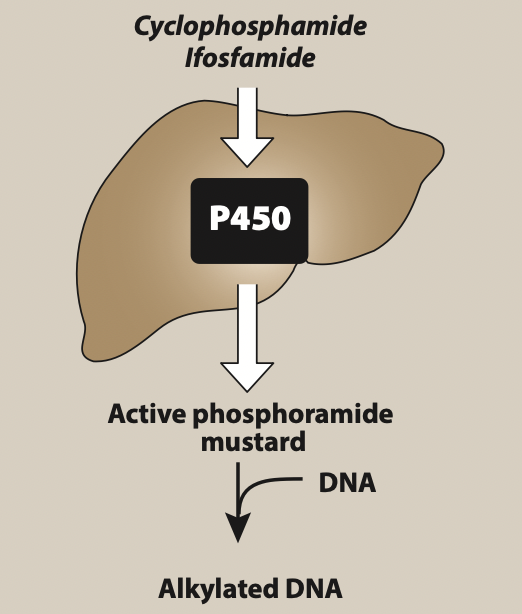
Figure- Activation of ifosfamide and cyclophosphamide (Source- Lippincott’s Illustrated Reviews)
- It is administered via IV route. When given by oral route, the bioavailability is around 100% but produces severe neurotoxicity. Hence, oral route is not preferred.
- It is metabolized mainly by CYP450 3A4 and 2B6 isoenzymes and is excreted via renal route. Its plasma half-life is around 15 hours when used in dose of 3.8-5 g/m2 and shorter half-life at low dose.
Adverse Effects
- One of the major dose limiting side effect is hemorrhagic cystitis. To prevent this, it is administered with Mesna, which is -SH compound and combines with active metabolites like acrolein to form non-toxic compound. This non-toxic compound is excreted via urine.
- Other side effects include alopecia, nausea, vomiting, abdominal cramps, anorexia, and encephalopathy. Antiemetics is used along with it to prevent vomiting. It can also cause metabolic acidosis, cardiac arrhythmia, hematuria, and liver injury. High dose ifosfamide may cause neurotoxicity due to byproduct chloroacetaldehyde.
- Compared to cyclophosphamide, it causes less alopecia and emetogenic effect.
Drug Interaction
- Co-administration with drugs like rifampicin, carbamazepine, phenytoin and phenobarbital which are CYP3A4 induces may increase its activation. CYP3A4 inhibitors like itraconazole, verapamil, cyclosporin and erythromycin decrease its activation.
Contraindications
- Contraindicated in patients with known hypersensitivity to ifosfamide.
- Contraindicated in patients with severe renal or hepatic impairment.
- Contraindicated in patients with myelosuppression.
References
- Essentials of Medical Pharmacology. 7th edition.
- Lippincott’s Illustrated Reviews, Pharmacology. 7th edition.
- Goodman and Gilman’s manual of Pharmacology and Therapeutics.
- https://www.ncbi.nlm.nih.gov/books/NBK542169/
- Zhang J et al. Clinical Pharmacology of Cyclophosphamide and Ifosfamide. Current Drug Therapy. 2006; 1(1).
- Kerbusch T et al. Clinical Pharmacokinetics and Pharmacodynamics of Ifosfamide and its Metabolites. Clinical Pharmacokinetics. 2001; 40: 41–62.