- Everolimus is macrolide derivative and belongs to class of mTOR (mammalian target of Rapamycin) inhibitor. Its indication includes immunosuppressant and oncological indications.
- It is derivative of sirolimus and has similar chemical and clinical properties but have different pharmacokinetic profiles. Everolimus was introduced to improve pharmacokinetic characters of sirolimus, mainly to increase its oral bioavailability.
Indication of Everolimus
- Used to prevent transplant rejection in kidney transplant patients in combination with cyclosporine, corticosteroids and basiliximab.
- Used in treating adult patients with progressive neuroendocrine tumors of pancreatic origin (PNET) with locally advanced or metastatic disease.
- In treatment of postmenopausal women with advanced hormone receptor positive, HER 2 negative breast cancers (advanced HR+ BC).
- Used in advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib.
- In adult patients with renal angiomyolipoma and tuberous sclerosis complex (TSC) who don’t require immediate surgery.
- In pediatric and adult patients with tuberous sclerosis complex (TSC) for treating subependymal giant cell astrocytoma (SEGA) which cannot be curatively resected but require therapeutic intervention.
Mechanism of action of Everolimus
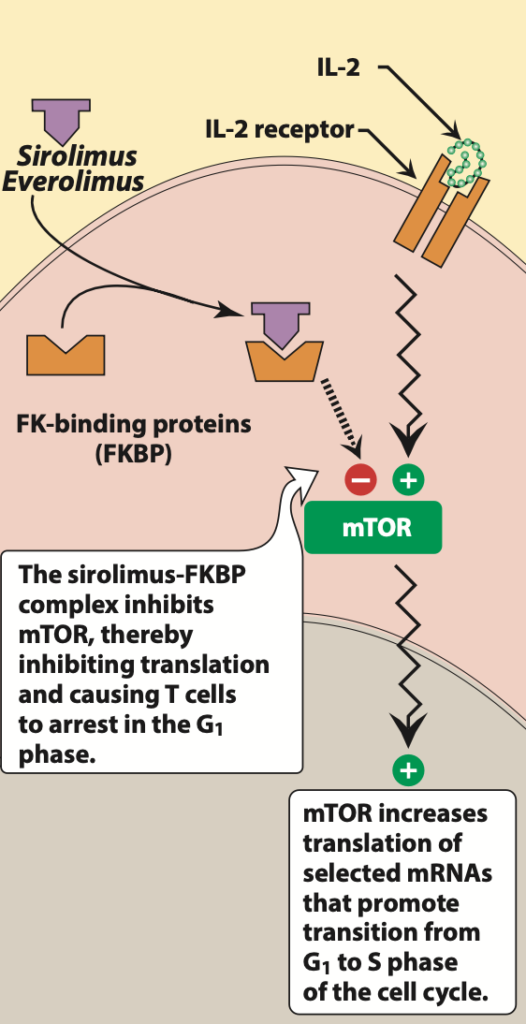
- Figure- Mechanism of action of Everolimus (Source- Lippincott’s Illustrated Reviews Pharmacology, 6th edition)
- It is mTOR inhibitor which first binds to immunophilin, FK binding protein- 12 (FKBP). The everolimus- FKBP 12 complex binds to and inhibits multifunctional, serine-threonine kinase, mTOR (mammalian target of Rapamycin). mTOR plays important role in various cellular activity like cell cycle progression, DNA repair and regulation of protein translation. Inhibition of mTOR results in blockade of progression of activated T cells from G1 phase to S phase and hence inhibits proliferation of activated T cells. Hence, it results in induction of cell growth arrest and apoptosis.
- It also inhibits expression of hypoxia inducible factor which results in decreased expression of vascular endothelial growth factor.
Pharmacokinetics of Everolimus
- It is rapidly absorbed after oral administration. Its bioavailability is decreased by high fat meal. Peak plasma concentration may be achieved within 1-2 hours of oral administration.
- It binds extensively to plasma proteins and circulating red blood cells. Hence, monitoring of whole blood trough concentration is recommended in patients on everolimus therapy. Like sirolimus, it is substrate of P-glycoprotein (P gp) and is metabolized in liver by CYP3A4 isoenzymes. Dosage reduction is required in patients with hepatic impairment. Less than 10% of drug is excreted unchanged in urine.
- Its half-life is shorter compared to sirolimus and require twice per day dosing.
Adverse effects
- Some common side effects include myelosuppression, especially thrombocytopenia, diarrhea, hepatoxicity and pneumonitis. Everolimus also causes increase in cholesterol and triglyceride level which may require treatment. It may cause impaired or delayed wound healing in obese or diabetic patients which may have severe effect immediately after the transplant surgery and in patients who are on corticosteroid therapy.
- Everolimus can cause angioedema. It may increase risk of kidney arterial and venous thrombosis which may result in graft loss, mostly in 30 days after organ transplantation.
Drug/ Food interactions
- When used with cyclosporine, it increases plasma drug concentration of cyclosporine by decreasing its metabolism and increase risk of nephrotoxicity. Hence, dose of cyclosporine should be reduced when used in combination with everolimus. Concurrent administration with ACE (angiotensin converting enzyme) inhibitors like captopril, enalapril may increase risk of angioedema.
- As it is substrate of CYP3A4, it may interact with other drugs which inhibit or upregulate CYP3A4 activity. Drugs which are CYP3A4 inhibitor like azole antifungal (itraconazole, fluconazole, tioconazole) and macrolide antibiotics may increase plasma concentration of everolimus. CYP3A4 inducer like rifampin may increase metabolism of everolimus.
- Grapefruit may inhibit metabolism of everolimus by CYP3A4 pathway and may increase its plasma concentration. St. John’s wort may induce CYP3A4 and decrease plasma concentration of everolimus.
Contraindications
- Contraindicated in patients hypersensitive to everolimus.
References
- https://go.drugbank.com/drugs/DB01590
- Huiras P et al. Everolimus: a review of its pharmacologic properties and use in solid organ. Reviews in Health Care. 2011; 2(4): 229-241.
- Heine R et al. A pharmacological rationale for improved everolimus dosing in oncology and transplant patients. Br J Clin Pharmacol. 2018 Jul; 84(7): 1575–1586.
- Houghton PJ. Everolimus. Clin Cancer Res. 2010 Mar 1; 16(5): 1368–1372.
- Hasskarl J. Everolimus. Recent Results Cancer Res. 2018; 211: 101-123.
- Kidney Transplantation, Bioengineering and Regeneration. Kidney Transplantation in the Regenerative Medicine Era. 2017, Pages 429-443.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.