- Paracetamol also known as acetaminophen is commonly used medicine for pain and fever. It belongs to para-amino phenol derivative group. It is included in WHO (World Health Organization)’s List of Essential Medicines and is available as generic medicine.
- Paracetamol was first synthesized in 1878 by Morse and was introduced in medical field in 1883. It is OTC (Over the Counter) drug. In 2017, it was 25th most commonly prescribed medicine in USA. More than 100 different preparation containing paracetamol alone or in combination with other drugs (e.g. caffeine, tramadol or NSAIDs) are available. It is inexpensive in most countries and is generally considered safe.
Mechanism of action of Paracetamol
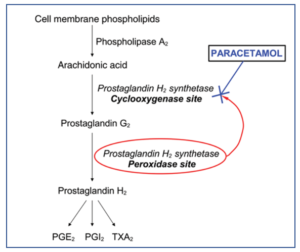
Figure 1- Role of paracetamol in inhibition of prostaglandin production (Source- Sharma et al, 2014)
- The complete mechanism of action of paracetamol is not completely understood.
- During pain, inflammation and fever, arachidonic acid is liberated from phospholipid fraction of cell membrane by phospholipase A2. Arachidonic acid is converted to prostaglandins (PGs) by cyclooxygenase (COX-1 and COX-2).
- The prostaglandins produced sensitize blood vessels to other inflammatory mediators which increase permeability and sensitize chemical receptor of afferent pain ending to mediators such as histamine and bradykinin. PGE2 and PGI2 produce hyperalgesia associated with inflammation. They are also involved in pyretic response.
- Paracetamol is weak inhibitor of synthesis of prostaglandins. It inhibits COX enzymes and thus inhibit prostaglandin synthesis in brain resulting in its analgesic and anti-pyretic activity.
- The inhibition of COX enzymes on peripheral inflamed tissues by paracetamol is weak (due to peripheral inactivation). This results in weak anti-inflammatory activity.
- There is some evidence which suggest that analgesic effect of paracetamol is due to its central action which is due to activation of descending serotonergic pathways.
Pharmacokinetics of Paracetamol
- It is available in oral, IV, IM and rectal formulation.
- When administered through oral route, it has excellent bioavailability, is absorbed rapidly and almost completely. Peak plasma concentration occurs within 30-60 minutes and half-life in plasma is about 2 hours.
- Metabolism takes place in liver and form inactive glucuronidated or sulfated metabolites. Some portion of paracetamol is hydroxylated to form N-acetyl-p-benzoquinoneimine (NAPQI), a highly reactive metabolite which can react with sulfhydryl groups and cause liver damage. In normal dose of paracetamol, NAPQI react with sulfhydryl group of glutathione and form non-toxic product.
- Paracetamol and its metabolites are excreted in urine. Around 90-100% of drugs may be recovered in urine within first day (24 hours) of therapeutic dosing.
Therapeutic Uses of Paracetamol
- It is suitable alternative for aspirin for analgesic and anti-pyretic uses and is useful for patients in whom aspirin is contraindicated (patients with peptic ulcer, aspirin allergy, children with febrile illness).
- For relief of mild to moderate pain including migraine, kidney stone pain, tension headache and perineal pain after childbirth.
- It is analgesic/antipyretic of choice in children with viral infection or chickenpox (due to chance of Reye’s syndrome with aspirin).
- To relieve osteoarthritic pain in elder patients.
- For treatment of patent ductus arteriosus (condition in preterm infants characterized by failure or delay in spontaneous closure of ductus arteriosus).
- Used in combination with caffeine. This is more effective than paracetamol alone in dental pain, postpartum pain and headache.
Adverse Effects
- Acetaminophen, in normal therapeutic dose, is well tolerated and has minimum side effects.
- The most serious adverse effect is hepatic damage. In large doses (˃6 gm), it can cause extensive hepatocellular damage, renal necrosis and death. Hepatic and renal damage is due to metabolite NAPQI, which is normally turned into non-toxic, harmless product by combining with glutathione. Depletion of hepatic and renal glutathione lead to reaction of NAPQI with sulfhydryl group of hepatic proteins and potentiate its toxicity.
- Patient with hepatic disease, viral hepatitis or history of alcoholism are at higher risk of hepatotoxicity due to paracetamol. In individuals with G6P deficiency, it can cause hemolysis and results in anemia.
- It can cause skin rashes, drug fever, neutropenia, thrombocytopenia and nephropathy. It may also cause nausea, vomiting and abdominal pain rarely.
Drug Interaction
- Concurrent administration with enzyme-inducing substances, such as carbamazepine, phenytoin, or barbiturates, as well as chronic alcohol excess, may increase NAPQI production and the risk of paracetamol toxicity. Isoniazid also increases its risk of toxicity when used together.
- It can interact with warfarin and other oral anti-coagulants and increase prothrombin time.
Contraindication
- It is contraindicated in patients allergic or sensitive to paracetamol.
- It is avoided in hepatic patients.
References
- Garry GG, Kieran SF. Mechanism of Action of Paracetamol. American Journal of Therapeutics. 2005; 12(1): 46-55.
- Bardanzellu F, Neroni P, Dessi A, Fanos V. Paracetamol in Patent Ductus Arteriosus Treatment: Efficacious and Safe? Hindawi BioMed Research International. 2017; 1438038: 1-25.
- Roberts E, Nunes VD, Buckner S, Latchem S, Constanti M, Miller P et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis. 2016; 75(3): 552–559.
- Saragiotto BT, Shaheed CA, Maher CG. Paracetamol for pain in adults. BMJ. 2019; 367: l6693.
- McCrae JC, Morrison EE, Maclntyre M, Dear JW, Webb DJ. Long‐term adverse effects of paracetamol – a review. Br J Clin Pharmacol. 2018; 84(10): 2218–2230.
- Sharma CV, Mehta V. Paracetamol: mechanisms and updates. Continuing Education in Anaesthesia Critical Care & Pain. British Journal of Anesthesia. 2014; 14(4): 153–158.
- Goodman and Gillman’s Manual of Pharmacology.
- Pharmacology and pharmacotherapeutics. 24th edition.
- A Textbook of Clinical Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology. 6th edition.