- Leukotriene Modifiers are medicines used to manage allergic rhinitis or allergy and asthma. They are also known as antileukotriene or leukotriene antagonist.
- They entered clinical practice in 1996-1997 in several countries including Japan, United states and Britain.
What are Leukotrienes?
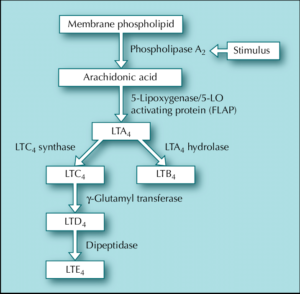
Figure 1- Synthesis of leukotrienes
- Leukotrienes (LT) are fatty acid derived mediators containing a conjugated triene structure.
- Arachidonic acid is liberated from cell membrane as a result of cell activation due to allergy or noxious stimuli. The enzyme 5’ lipoxygenase is required for synthesis of LTA4, from arachidonic acid, which is unstable epoxide and acts as precursor for 2 subgroups of biologically important leukotrienes- LTB4 and cysteinyl leukotrienes.
- LTB4 is potent chemoattractant for neutrophils and eosinophils. Cysteinyl leukotrienes (LTC4, LTD4 and LTE4) cause constriction of bronchial smooth muscle, promote mucus secretion and mucosal edema, increase bronchial hyperresponsiveness and increase endothelial permeability.
- They play important role in pathophysiology of asthma.
Types of Leukotriene modifiers
- Leukotriene modifiers can be divided into 2 types based on their mechanism of action.
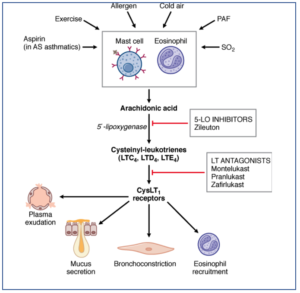
- Figure- Mechanism of action of two types of leukotriene modifiers
1) Leukotriene synthesis inhibitor:
- Zileuton is an example of leukotriene synthesis inhibitor.
- It acts by potent and selective inhibition of 5-lipooxygenase enzyme and hence, inhibits formation of both LTB4 and cysteinyl leukotrienes.
2) Cysteinyl leukotriene receptor antagonist
- Examples are Montelukast and zafirlukast.
- Cysteinyl leukotrienes were collectively termed as ‘slow reacting substance of anaphylaxis (SRS-A). They are 10-5000 times more potent than other bronchoconstrictor agents such as histamine, platelet activating factor or prostanoids.
- Cysteinyl leukotrienes (CysLTs) bind to CysLT1 receptor to produce bronchoconstrictor effect. Zafirlukast and montelukast are selective, high-affinity competitive antagonist for CysLT1 receptor and result in inhibition of CysLT1 induced bronchial smooth muscle contraction. The result is the relief from symptoms of asthma.
Pharmacokinetics of Leukotriene Modifiers
- All three drugs are orally active and highly protein bound. Food impairs absorption of zafirlukast.
- Extensive metabolism takes place in liver.
- Zileuton and its metabolites are excreted in urine whereas montelukast and zafirlukast and their metabolites are excreted through bile in feces.
| LT modifier | Bioavailability | Half-life (Hours) | Excretion |
| Montelukast | ̴60-70 % | 3-6 | through bile |
| Zafirlukast | ̴90% | ̴10 | through bile |
| Zileuton | ̴93% | ̴2 | through urine |
Table 1- Pharmacokinetic parameters of leukotriene modifiers
Therapeutic Uses of Leukotriene Modifiers
- To treat exercise induced bronchoconstriction. They can be used as an alternative to low dose inhaled corticosteroids or when side effects, poor inhaler administration technique, non-compliance is suspected.
- In aspirin induced bronchospasm.
- Used in cold air induced bronchoconstriction.
- In allergic rhinitis.
- In mild to moderate chronic persistent asthma.
They have no bronchodilator action, hence cannot be used as rescue drugs when immediate bronchodilation is required. They are typically not a first-line treatment in any of above-mentioned cases. However, they can be used as alternative to low dose inhaled glucocorticoid therapy in mild asthma in children. They are expensive and their superiority above older drugs used for asthma like beta-2 adrenergic receptor agonists, aminophylline is not established. Hence, they are used only in selective cases.
Montelukast has several advantages over other LTRAs, including formulation, onset of action, duration of action, and a low incidence of adverse effects. Perhaps most important, chronic daily use does not result in the development of tolerance. So, it is preferred more.
Adverse Effects
- They are generally well tolerated.
- Some common side effects are headache, gastrointestinal disturbances, dyspepsia, nausea, vomiting, rashes and fever. All 3 agents can cause elevation of serum hepatic enzymes. So, regular monitoring of hepatic enzymes is required.
- They can also cause pulmonary eosinophilia, hallucinations, sleep disorders and seizures. They are very rare.
- Agitation symptoms may occur after drug withdrawal.
Drug Interaction
- Zilueton decreases clearance of drugs like theophylline, propranolol and warfarin.
- Zafirlukast may interact with warfarin and increase prothrombin time. It may also interfere with antiepileptic drugs like phenytoin and carbamazepine.
Contraindication
- Contraindicated in patients who are allergic or hypersensitive to them.
- Zileuton is contraindicated in patients with active liver disease or in whom hepatic enzymes level are three times the upper limit of normal.
References
- Philteos GS, davis BE, Cockcroft DW, Marciniuk DD. Role of Leukotriene Receptor Antagonists in the Treatment of Exercise-Induced Bronchoconstriction: A Review. Allergy Asthma Clin Immunol. 2005; 1(2): 60–64.
- Mali RG, Dhake As. A review on herbal antiasthmatics. Orient Pharm Exp Med. 2011; 11(2): 77–90.
- Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM. Leukotriene receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis. Ann Intern Med. 2015; 163(10): 756–767.
- Sampson A, Holgate S. Leukotriene modifiers in the treatment of asthma Look promising across the board of asthma severity. BMJ. 1998; 316(7140): 1257–1258.
- Hon KLE, Leung TF, Leung AKC. Clinical effectiveness and safety of montelukast in asthma. What are the conclusions from clinical trials and meta-analyses? Drug Des Devel Ther. 2014; 8: 839–850.
- A textbook of clinical pharmacology and therapeutics.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology. 6th edition.