- Atropine is tertiary amine belladonna alkaloids which possess anti-muscarinic effects. It is found in many plants belonging to family Solanaceae like Atropa belladonna, Datura stramonium and Hyoscyamus niger.
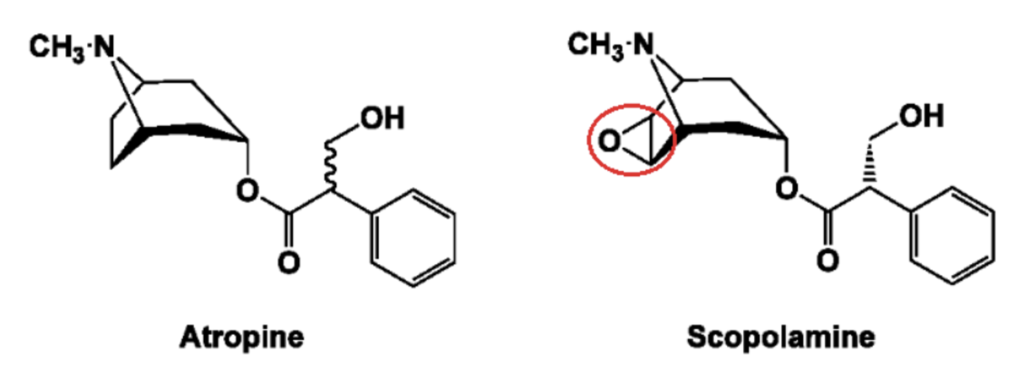
- It has high affinity towards muscarinic receptors. Atropine or dl-hyoscyamine is an ester of an aromatic organic acid ‘tropic acid’ with a complex organic base ‘tropine’.
- Another important belladonna alkaloid is scopolamine (hyoscyamine) which is an ester of ‘tropic acid’ with organic base ‘scopine’. Scopolamine has similar mechanism of action and pharmacological actions as of atropine. However, it action on CNS is different than of atropine.
- Atropine is included in World Health Organization’s list of essential medicines.
Mechanism of Action of Atropine
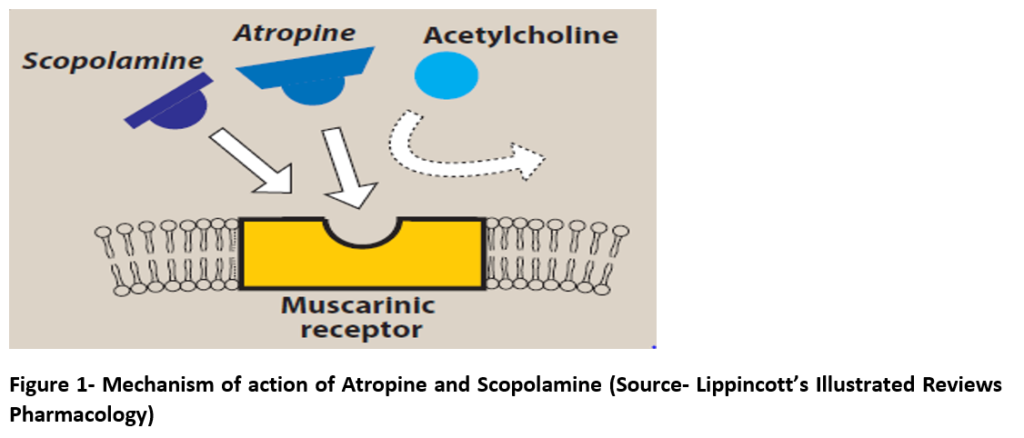
- Atropine competes with acetylcholine (ACh) to bind with muscarinic receptors and prevent ACh from binding to those sites resulting in blockade of both peripheral and central muscarinic effects of Ach.
- Atropine is more effective in blocking effects of externally administered ACh than the effects of cholinergic nerve stimulation.
- The dose of atropine required to muscarinic blockade varies according to organ. Salivary and bronchial secretions are affected even by small dose of atropine whereas eye, heart and gastric secretion are less affected even after administration of large doses.
- Atropine can block nicotinic action of ACh at the autonomic ganglia in large doses.
Pharmacological Actions of Atropine
Secretions
- Atropine reduces secretion of exocrine glands except milk production, blocks saliva secretion and cause dryness of mouth and difficulty in swallowing.
- It reduces secretion in nose, mouth, pharynx and bronchi. It has little effect on pancreatic and intestinal secretions.
Gastrointestinal Tract
- Atropine reduces tone and motility of gut, thus can be used as antispasmodic. Its effect on vagus nerve stimulation is partial so its effect on normal peristalsis is not significant.
- It reduces volume and total acidity of gastric secretion.
Eye
- Atropine on local instillation blocks cholinergic response of smooth muscle of sphincter of iris and causes mydriasis (dilation of pupil).
- It paralyses ciliary smooth muscle which control lens curvature and thus cause cycloplegia (paralysis of accommodation).
- In individuals with narrow angle glaucoma and shallow anterior chamber, it may increase intraocular tension dangerously.
Cardiovascular System
- Its action on heart varies according to dose.
- In therapeutic doses, it initially decreases the heart rate which is due to blockade of M1 receptors on inhibitory presynaptic neurons which is followed by tachycardia. It also completely blocks vasodilation and hypotension produced by cholinergic agents in therapeutic doses.
- In large doses, it produces increase in heart rate due to blockade of M2 receptors in SA (Sinoatrial) node.
Central Nervous System
- In therapeutic doses, it stimulates medulla and higher cerebral center and results in mild vagal excitation. This occasionally produces bradycardia and increases rate and depth of respiration.
- In toxic doses, it produces more prominent central excitation and causes restlessness, irritability, hallucination and delirium. With still large doses, excitation is followed by depression leading to respiratory failure and circulatory collapse.
Pharmacokinetics of Atropine
- It is absorbed rapidly from GI tract, parenteral sites and from mucous membranes. Absorption from intact skin is limited.
- Atropine is partly metabolized in liver and partly excreted unchanged in urine. It has t1/2 of about 4 hours.
- It crosses the BBB (Blood Brain Barrier), placental barrier and is secreted in milk and saliva.
Therapeutic Uses
- Used as an antispasmodic agent to relax the GI tract.
- In treatment of organophosphorus poisoning, some types of mushroom poisoning (some mushroom contain cholinergic substances that block cholinesterase) and overdose of clinically used anticholinesterases like physostigmine.
- Used as an antidote for cardiovascular collapse caused due to choline ester (cholinergic drugs).
- Useful in abolishing AV-block due to excessive vagal activity.
- To overcome severe bradycardia and syncope.
- Used as pre-anesthetic medication to prevent or reduce respiratory secretions.
- It is used to produce mydriasis and cycloplegia. Mydriasis is necessary for a fundoscopic examination and in the treatment of acute iritis, iridocyclitis and keratitis.
Note- Atropine derivatives and substitutes are preferred than atropine in most of above-mentioned conditions. Atropine has retained its therapeutic use in treatment of AV-block and organophosphorus poisoning.
Adverse Effects
- Adverse effects including dry mouth, blurred vision, sandy eyes, tachycardia, constipation are dose dependent.
- Effect on CNS includes restlessness, agitation, delirium which may progress to depression, respiratory and circulatory system collapse and finally death.
- Infants and young children are more susceptible to CNS toxicity. In elder people, it can precipitate glaucoma and urinary retention.
- Acute atropine poisoning may occur.
- When applied locally, allergic reactions such as dermatitis, conjunctivitis and swelling of eyelids may occur.
Contraindications
- Contraindicated in patients with known atropine or belladonna alkaloids hypersensitivity.
- Used with caution in patients with cardiac dysfunction or a recent myocardial infarction.
- Used with caution in patients with enlarged prostate (as urinary retention may develop) and with chronic lung conditions (as it causes dryness and reduce secretions).
- Should be used with caution in patients over age 80 as it may precipitate an attack of acute angle glaucoma.
Reference
- Toxicological Aspects of Drug-Facilitated Crimes. 2014: Pages 47-91.
- Medical Emergencies in the Dental Office (Seventh Edition). 2015: Pages 62-112.
- Handbook of Toxicology of Chemical Warfare Agents. 2009: Pages 985-996.
- Goodman and Gillman’s Manual of Pharmacology.
- Pharmacology and pharmacotherapeutics. 24th edition.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- https://www.ncbi.nlm.nih.gov/books/NBK470551/