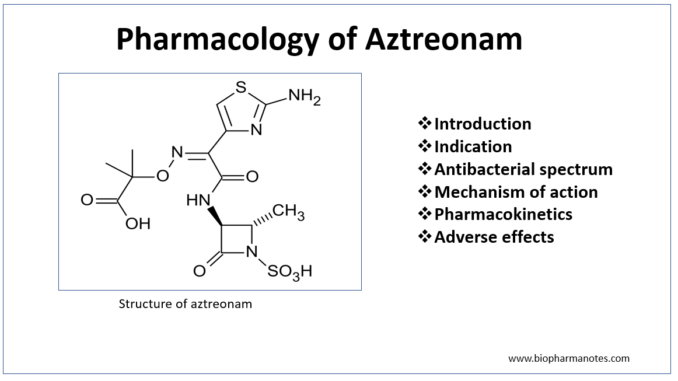
- Aztreonam is first monocyclic β- lactam antibiotics approved for use in clinical medicine. It belongs to monobactam class. Monobactams are unique compared to other β- lactam antibiotics as β- lactam ring is not fused to another ring.
- Aztreonam is only commercially available monobactam. The name monobactam is derived from ‘mono’- single ring, ‘bact’- produced by bacteria and ‘am’- beta lactam. Aztreonam is produced naturally by bacteria- Chromobacterium violaceum. It can also be produced synthetically.
- It is available as generic medicine.
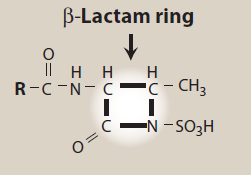
Figure- Structural feature of aztreonam (Source- Lippincott’s Illustrated Reviews)
Indications of aztreonam
- It is used in infections caused by susceptible gram- negative bacteria- urinary tract infection, lower respiratory tract infections, intra- abdominal infections, septicemia, skin infections and gynecological infections.
- Used as an alternative to amino-glycoside in severe infections caused by gram- negative bacteria like complicated UTI.
- In patients allergic to penicillin and cephalosporin.
- Used in treating neonatal gram- negative infection with ampicillin.
Anti-bacterial activity of aztreonam
- Its anti- bacterial activity is similar to that of aminoglycosides. It is effective only against gram- negative aerobic bacilli including P. aeruginosa and Enterobacteriaceae. It is highly active against gonococci and H. influenza.
- Aztreonam is not effective against gram- positive bacteria and anaerobes.
Mechanism of action
- It is a bactericidal drug which acts by inhibiting bacterial cell wall synthesis. It binds to penicillin binding proteins 3 (PBP3) and inhibits and 3rd and last stage of bacterial cell wall synthesis. This inhibition weakens the bacterial cell wall and makes bacteria vulnerable to rupture by solutes in surrounding medium.
- Autolytic enzymes present in bacterial cell wall like autolysins may mediate bacterial cell lysis.
Pharmacokinetics
- It is mostly administered via IV or IM route. when administered through oral route, less than 1 % is absorbed so oral route is not preferred.
- Its elimination half-life is around 1.7 hours. Most of the drug is excreted unchanged in urine. Around 6- 16 % is metabolized to inactive metabolites by hydrolysis of β- lactam ring and results in open ring compound. It is resistant to hydrolysis by most β- lactamases.
- The usual dose is 1-2 gm for every 8- 12 hours. Higher dose may be needed in P. aeruginosa infection and in lung infection in patients with cystic fibrosis.
- It is widely distributed throughout the body. It gets accumulated in patients with renal failure, so dose adjustment is required in renal patients.
Adverse effects
- Its safety profile is very good. There is no risk of ototoxicity and nephrotoxicity like aminoglycosides and routine monitoring of serum is also not required. It has weak immunogenic potential and not associated with coagulation abnormalities.
- Common side effects include nausea, vomiting, rashes, diarrhea, pain and swelling at the site of injection. Some other rare side effects are jaundice, loss of appetite, dark urine, burning eyes, severe stomach pain etc.
- It has lower risk of cross sensitivity with other β- lactam antibiotics. Ceftazidime- a cephalosporin is exception as both have similar side chain.
- It is pregnancy category B drug which means there is no evidence of risk in human, but studies are not adequate.
References
- https://go.drugbank.com/drugs/DB00355
- Duma RJ. Microbiology and pharmacology of aztreonam. Urology. 1988 Jun;31(6 Suppl):9-13.
- Neu HC. Aztreonam activity, pharmacology, and clinical uses. Am J Med. 1990 Mar 23;88(3C):2S-6S; discussion 38S-42S.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Essentials of Medical Pharmacology. 7th edition.
- Rang and Dale’s Pharmacology. 9th edition.