- Fluconazole is member of triazole antifungal agents, one of the commonly used antifungal agents. It is the first member of triazole class of antifungal agents which got its FDA approval in 1990.
- Fluconazole was discovered by Richardson et al in UK. It is included in World Health Organization’s List of Essential Medicines and is also available as generic medicine.
- It is semi-synthetic azole.
Indications of fluconazole
- Used to treat various fungal infections including:
- Systemic candida infections.
- Vaginal yeast infection caused by Candida.
- Cryptococcal meningitis.
- Peritonitis caused by Candida.
- Oropharyngeal and esophageal candidiasis.
- It is used prophylactically to prevent risk of candidiasis in patients undergoing bone marrow transplantation who receive radiation or cytotoxic chemotherapy.
- Some of its non-FDA approved uses include histoplasmosis, blastomycosis and coccidioidomycosis.
Mechanism of action of fluconazole
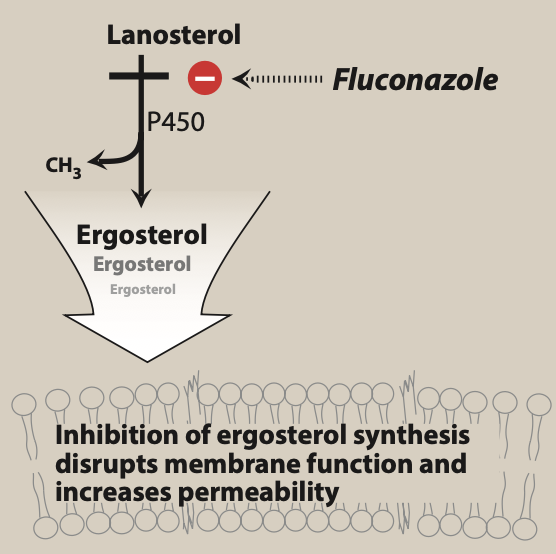
Figure- Mechanism of action of fluconazole (Source- Lippincott’s Illustrated reviews Pharmacology, 6th edition).
- It acts by inhibiting ergosterol synthesis. Ergosterol is important part of fungal cell membrane.
- It binds to and inhibits fungal cytochrome P450 enzyme, C-14 a-demethylase which is involved in 14- alpha demethylation reaction of lanosterol to ergosterol. This inhibition in ergosterol synthesis cause impairment in membrane structure and function and results in inhibition of fungal cell growth.
- It also prevents endogenous respiration and formation of yeasts.
Antimicrobial spectrum
- It is very effective against Candida and Cryptococcus species including Candida albicans, Cryptococcus neoformans etc. However, it is less effective against C. glabrata and has no activity against C. krusei at al.
- It is not effective against aspergillosis, mucormycosis and other true pathogenic fungi.
Pharmacokinetics of fluconazole
- It is administered via oral or IV route (IV infusion). Bioavailability of fluconazole is not affected by food or gastric pH. It undergoes extensive absorption in GI tract after oral administration.
- Protein binding property is low (around 11-12%). Metabolism occur in liver and undergoes renal excretion. Around 90% of drug is excreted unchanged in urine.
- It can penetrate CSF (cerebrospinal fluid) easily. It inhibits hepatic enzymes CYP3A4, CYP2C9 and CYP2C19. And it is distributed widely in body fluids including saliva,, sputum, CSF and breast milk and tissues.
Adverse effects of fluconazole
- Common side effects include GI disturbances, nausea, headache. It can cause elevation of hepatic enzymes. Due to high chances of having nausea and vomiting, patients who are receiving high dose of fluconazole (800 mg per day) should be given parenteral antiemetics.
- When used in high doses, it can cause skin rashes, muscle weakness and alopecia.
Drug Interactions
- As it inhibits hepatic enzymes CYP3A4 and CYP2C9, it inhibits metabolism of drugs which are substrate of these enzymes and increase their plasma concentration. Such drugs include phenytoin, cyclosporine, tacrolimus, sulfonylurea, theophylline, warfarin and cisapride.
- Rifampin decreases AUC (area under curve) of fluconazole by around 25%.
Contraindications
- Should be used with caution in patients with hepatic dysfunction and renal impairment.
- It is pregnancy category D drug. It can cause cardiac and skeletal deformation in babies if mother takes high dose of fluconazole during pregnancy. Hence, it is contraindicated in pregnancy.
- Contraindicated in patients hypersensitive to fluconazole.
- Used with caution in patients with pro arrhythmic conditions,
References
- https://www.ncbi.nlm.nih.gov/books/NBK537158/
- https://go.drugbank.com/drugs/DB00196
- A textbook of Clinical Pharmacology and therapeutics. 5th edition.
- Lippincott’s Illustrated Reviews Pharmacology. 6th edition
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Charlier C et al. Fluconazole for the management of invasive candidiasis: where do we stand after 15 years? Journal of Antimicrobial Chemotherapy. 2006; 57(3): 384-410.