- Sulfonamides are antimicrobial compound containing a sulfonamide group (SO2NH2). This group is also present in other non-antimicrobial compounds like antidiabetic sulfonylurea, thiazide diuretics, furosemide and acetazolamide.
- Structurally, they are derivatives of para-aminobenzene sulfonamide (sulfanilamide).
- Sulfonamide was firstly noted as anti-bacterial in 1900’s by Gerhard Domagk; a Nobel Prize winner in 1939. They were first broadly effective antibacterial to be used systemically which bring antibiotic revolution in medicine. Nowadays, they are rarely used in developed countries. However, they are still common antimicrobial medicine in developing countries due to their lower price.
Classification of sulfonamide
Clinically, the sulfonamides can be classified as:
- Those used for the treatment of systemic infections. They are subdivided into following:
Short acting– Sulfadimidine (sulfamethazine), Sulfacetamide, Sulfa-furazone (sulfisoxazole), and Sulfamethizole.
Intermediate acting: Sulfamethoxazole.
Long acting: Sulfadoxine, Sulfa-methoxy pyridazine, Sulfadimethoxine, Sulformethoxine.
- Those employed orally for the treatment of ulcerative colitis: Sulfasalazine.
- Those used topically: Mafenide, Silver sulfadiazine, Sulfacetamide.
Mechanism of action of sulfonamide
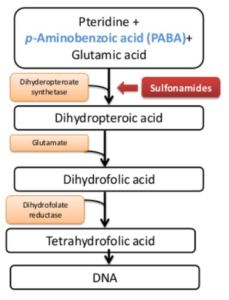
Figure- Mechanism of action of sulfonamides
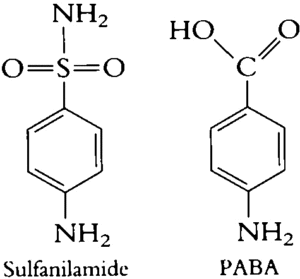
Figure- structure of sulfanilamide and para-aminobenzoic acid
- In many microorganism, dihydrofolic acid is synthesized from para-amino benzoic acid (PABA), pteridine and glutamate. This folic acid plays important role in bacterial metabolism and is essential for nucleic acid formation.
- The compound sulfanilamide possesses a structural similarity with para-amino benzoic acid (PABA). Due to this structural similarity, sulfonamide compete with PABA for enzyme dihydropteroate synthetase which is involved in conversion of PABA to dihydrofolic acid.
- Thus, they inhibit synthesis of dihydrofolic acid resulting in inhibition of formation of its essential cofactor forms. This results in injury to bacterial cell and bacteriostatic activity.
- Sulfonamide are effective in presence of pus and tissue breakdown products which contain large amount of PABA.
Anti-bacterial spectrum of sulfonamide
- Sulfonamides have wide antimicrobial activity. They are mainly bacteriostatic but may act as bactericidal in very high concentration.
- Sulfonamides are effective against various gram-positive and gram-negative organisms and certain chlamydia. These includes:
- Streptococci, Staphylococci (some strains), Gonococci, Pneumococci and Meningococci. Streptococcus fecalis is resistant.
- Clostridia, Bacillus anthracis.
- Haemophilus influenzae, H. ducreyi, Vibrio cholerae, E. coli, Pastuerella pestis, Shigella, Donovania granulomatosis.
- Nocardia and Actinomyces.
- Chlamydia organisms causing lymphogranuloma venereum, psittacosis and trachoma.
- When used in combination with dihydrofolate reductase inhibitor, they are effective against protozoal infections like toxoplasmosis and malaria.
Sulfonamide resistance
- Bacteria that can obtain folate from their natural environment shows natural resistance to sulfonamide.
- Acquired resistance to sulfonamide can arise from;
- Transfer of R plasmid factor.
- Increased production of natural substrate, PABA.
- Altered dihydropteroate synthetase.
- Decreased cellular permeability to sulfa drugs.
- An alternative metabolic pathway for synthesis of essential metabolite.
- Resistance in all enterobacteria is now common and in Shigella somnei is almost invariable. Other microorganisms such as Staphylococci, Streptococci, Pneumococci, Meningococci and coli can develop resistance.
Pharmacokinetics
Absorption
- Most sulfonamides are well absorbed after oral administration and 70-90% of dose reaches the blood circulation. Exception is sulfasalazine which is used to treat chronic inflammatory bowel disease.
- IV sulfonamide is used in patients who cannot take oral preparation. Absorption through vaginal route, respiratory tract and abraded skin is variable, but sufficient amount is absorbed to produce sensitization and toxicity.
Distribution
- They bind to plasma proteins, especially albumin. Various compounds differ in their degree of binding. In general, at least 50 % of the compound is bound to plasma proteins.
- The bound sulfonamide cannot pass into tissue fluid and cannot cross BBB and are not available for renal excretion. Thus, protein binding helps to prolong their action.
- Their free from gets distributed throughout body fluid and can penetrate CSF (cerebrospinal fluid) even in absence of inflammation. They can cross placental barrier and are secreted in milk.
Metabolism
- They are metabolized in liver by acetylation and conjugation. The major derivative is N4-acetylated sulfonamide. Acetylated metabolite has no antibacterial activity but retain toxic potential of parent compound.
Excretion
- Free and acetylated sulfonamide are mainly excreted in urine through glomerular filtration. Small amounts are excreted in feces, bile, milk and other secretions. Excretion of long acting and intermediate acting sulfonamide is slow.
Therapeutic Uses
- Sulfonamides are cheap, broad spectrum chemotherapeutic agents. because of resistance in different microorganisms, their use is declined nowadays. So, they are used in combination with trimethoprim.
- Used in urinary tract infection.
- In IBD (Inflammatory Bowel Disease).
- Used in combination with pyrimethamine to treat resistant toxoplasmosis and malaria.
- Also used in combination with trimethoprim to treat acute bacillary dysentery.
- To treat meningococcal infection, burn therapy and dermatitis herpetiformis.
- Effective in trachoma, conjunctivitis and superficial ocular infection. Though effective, it is no more drug of choice.
- Sulfadiazine in combination with antibiotic is useful in nocardiosis. Sulfasalazine is useful in rheumatoid arthritis.
Adverse Effects
- Allergy or hypersensitivity reactions like rashes, drug fever, eosinophilia, angioedema is common. Long acting sulfonamides can cause Steven Johnson syndrome and toxic epidermal necrolysis. Serum sickness characterized by joint pain, fever, urticaria and bronchospasm may occur within first two weeks of therapy.
- Renal toxicity– Nephrotoxicity can occur due to crystalluria. Renal damage with vasculitis and tubular necrosis can result due to allergic reaction.
- Hemopoietic toxicity– They oxidize hemoglobin to methemoglobin and can cause thrombocytopenia, granulocytopenia and aplastic anemia. They may cause hemolysis in patients with G6P deficiency.
- Bilirubin metabolism or kernicterus– Sulfonamides when administered during pregnancy, displace bilirubin from albumin binding sites in fetus. The free bilirubin crosses BBB of fetus as it is not well developed and cause kernicterus.
- Nervous system toxicity– CNS disturbance includes fatigue, tinnitus, ataxia, confusion, psychotic episodes and peripheral neuritis (rarely).
- Miscellaneous– Nausea, vomiting and anorexia may occur in 1-2 % cases. Rare chances of hypothyroidism and goiter persist.
Drug Interaction
- Sulfonamides may displace drugs like sulfonylurea, coumarin anticoagulant (warfarin), phenytoin and methotrexate from plasma protein binding sites and enhance their activity and serum level.
- Drugs like salicylate and phenylbutazone may displace sulfonamide from binding sites and increase their serum level and toxicity.
- Drugs containing PABA in their structure like procaine (local anesthetic) cause direct inhibition of sulfonamide activity.
Contraindication
- Contraindicated in neonates and pregnant women.
- It should not be used by nursing mothers.
- Contraindicated in person with sulfonamide allergy.
References
- Smith CL, Powell KR. Review of the Sulfonamides and Trimethoprim. Pediatrics in Review. 2000; 21 (11): 368-371.
- Dorn JM. Alpern M, McNulty C, Volcheck GW. Sulfonamide Drug Allergy. Curr Allergy Asthma Rep. 2018; 18(7): 38.
- R Lavanya. Sulphonamides: A Pharmaceutical Review. International Journal of Pharmaceutical Science Invention. 2017; 6(2): 01-03.
- Yousef F, Mansour O, herbali J. Sulfonamides: Historical Discovery Development (Structure-Activity Relationship Notes. In-vitro In-vivo In-silico Journal. 2018; 1(1): 1-15.
- The Journal of Allergy and Clinical Immunology: In Practice. 2019; 7(7): 2116-2123
- Lippincott Illustrated Reviews Pharmacology. 6th edition
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.