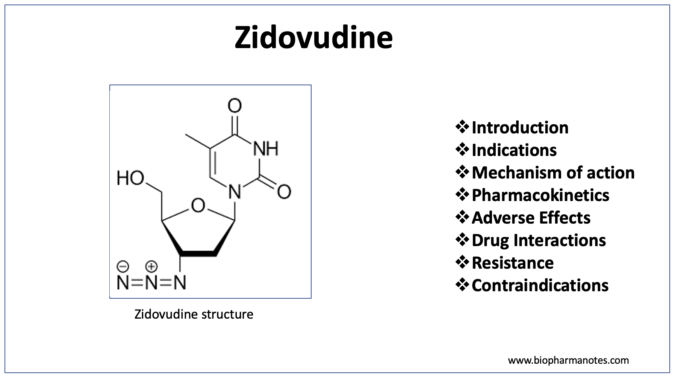
- Zidovudine which is also known as azidothymidine belongs to class of drug called Nucleoside reverse transcriptase inhibitor (NRTIs). Chemically, it is a dideoxynucleoside compound in which 3’ hydroxy group of sugar moiety is replaced by an azido group.
- It was synthesized in 1964 for use as chemotherapeutic agent and is the first nucleoside analogue to be used in treatment of HIV-1 (Human Immunodeficiency Virus-1) infection. Zidovudine is included in World Health Organization’s List of Essential Medicines.
- Other FDA approved NRTIs include stavudine, didanosine, tenofovir and lamivudine.
Indications of zidovudine
- For treating adult and child with HIV-1 infection. It is used in combination with other anti-retroviral agents including non- nucleoside reverse transcriptase inhibitors (NNRTIs) or protease inhibitors (PIs).
- For preventing mother to child transmission of HIV infection.
- Used as post- exposure prophylactic agent in HIV exposed healthcare workers.
Mechanism of action of zidovudine
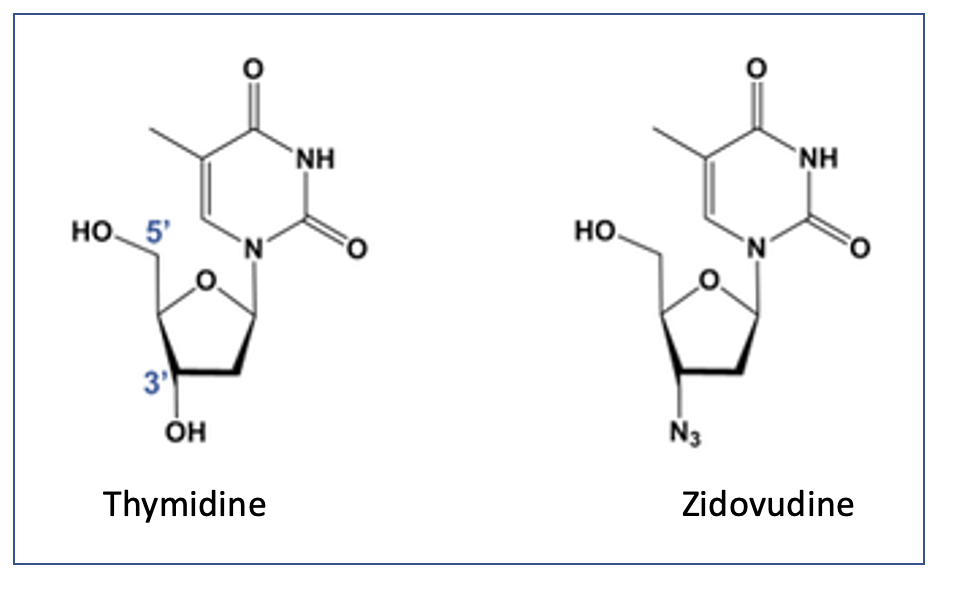
Figure- Structural similarity between zidovudine and thymidine
- It is a structural analogue of thymidine with azido group in place of hydroxyl group at 3’ position of deoxyribose ring. It is a prodrug which is phosphorylated at first to zidovudine monophosphate (ZDV-MP), then to zidovudine diphosphate (ZDV-DP) which is than phosphorylated to active metabolite, zidovudine triphosphate (ZDV-TP). ADV- To is structurally similar to thymidine triphosphate which is normally produced by cells and is one among nucleoside compounds required to synthesize DNA.
- ZDV-TP is competitive inhibitor of HIV-1 reverse transcriptase (RT) which competes with natural substrate- deoxythymidine triphosphate (dTTP) and is incorporated into viral DNA by RT. As, it doesn’t have 3’- hydroxyl group, a 3’,5’- phosphodiester bond cannot be formed between incoming nucleoside triphosphate and hence, growing DNA chain cannot be formed. This results in termination of viral DNA chain elongation.
- Human cell doesn’t have reverse transcriptase and zidovudine has less affinity for human DNA polymerases compared to HIV reverse transcriptase. Hence, ZDV-TP exert selective effect on viral replication.
Pharmacokinetics of zidovudine
- It is well absorbed after oral administration. Around 90% drug is absorbed from GI tract after oral administration. It is also administered via IV route.
- It can penetrate cerebrospinal fluid (CSF) and amniotic fluid. Its half-life is around 1 hour. The CSF concentration is around 50% of plasma concentration.
- It is metabolized in liver via glucuronidation and is excreted via urine. The amjor metabolite (around 80%) is glucuronide.
Adverse effects
- It can cause dose dependent bone marrow suppression which can cause anemia, granulocytopenia and reticulocytopenia.
- It can also cause headache, GI upset (nausea and vomiting), fatigue, myalgia, insomnia and melanonychia (blue- grey nail discoloration) which are generally resolved within first few weeks of therapy. Some rare side effects include agitation, hepatotoxicity, hepatic steatosis (accumulation of fat in liver cells) with lactic acidosis (accumulation of lactic acid in body fluids) and mitochondrial myopathy.
Drug Interactions
- Its metabolism (glucuronidation) is reduced by drugs like atovaquone and probenecid. Its metabolism is increased by drugs like rifamycin.
- Concurrent administration with ganciclovir increases risk of bone marrow suppression. Concurrent administration of zidovudine with anti-tuberculosis drugs incrase risk of anemia.
Resistance
- HIV virus and mutate and develop resistance against zidovudine. To prevent this and overcome drug resistance, it is used in combination with at least two or three other anti-retroviral agents.
- Patients on combination therapy of zidovudine and other agents should be monitored closely for plasma HIV RNA concentration to check the efficacy of drugs.
Contraindications
- Contraindicated in life-threatening hypersensitivity to zidovudine or any other components of the formulation
References
- https://go.drugbank.com/drugs/DB00495
- https://www.britannica.com/science/AZT
- D’ Andrea g et al. AZT: an old drug with new perspectives. Curr Clin Pharmacol. 2008 Jan;3(1):20-37.
- Primary Care Update for OB/GYNS. 1996; 3(2): 58-62.
- Demir M et al. Neurotoxic effects of AZT on developing and adult neurogenesis. Front. Neurosci. 2015; 9(93): 1-15.
- Ghodke y et al. PharmGKB summary: zidovudine pathway. Pharmacogenet Genomics. 2012 Dec; 22(12): 891–894.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- A textbook of Clinical Pharmacology and Therapeutics. 5th edition