Introduction
Chimeric Antigen Receptor (CAR) T cell Therapy is a type of Adoptive Cell Therapy (ACT) in which T-cells are modified and engineered to recognize antigen present in tumor cells without being presented via MHC (Major Histocompatibility Complex) in contrast to convention TCR mediated response. T cells are engineered to recognize tumor antigen only by using antigen-specific inhibitory CAR (iCAR). CARs capable of identifying the unprocessed antigens including glycolipid and carbohydrate structure that are expressed on the tumor cell surface. Since, it does not require processing of antigen, both CD8+ and CD4+ T-cells can be used to recognize the target cell. Since, CAR T-cell therapy used T-cell, it also falls under immunotherapy and called as “living therapy’.
The development of CARs includes following:
- scFv (single-chain fragment variable), derived from antibodies- the scFv is a variable monoclonal antibody fragment, derived from mouse monoclonal antibodies (mAbs), humanized Abs or fully human Abs. scFv part of CAR recognize tumor-associated antigens (TAAs), expressed on the tumor cell surface and bind to it.
- a human Fab fragment, selected from phage display libraries; or
- nature ligands that engage their cognate receptor
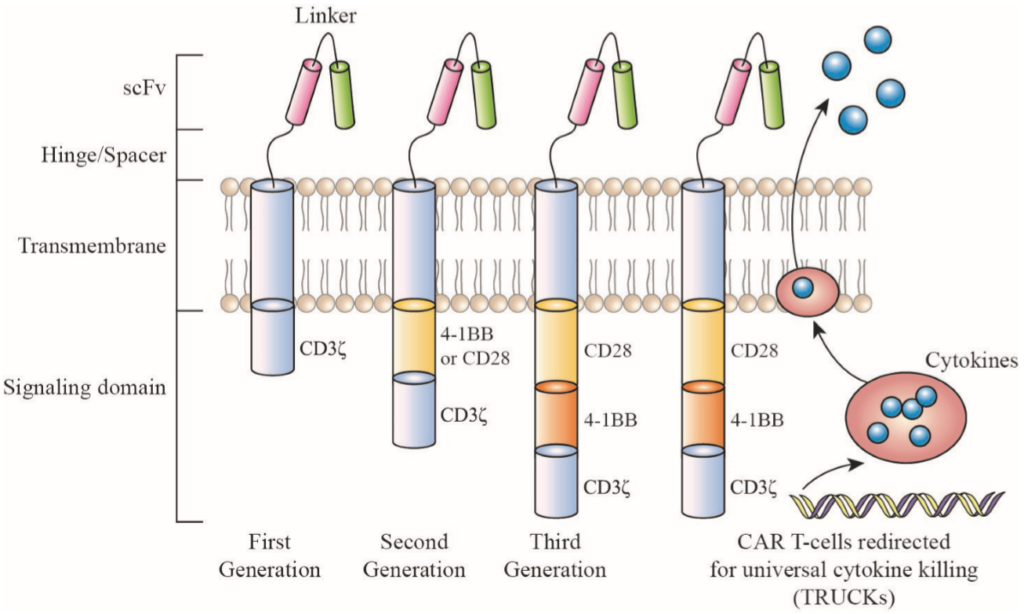
Figure 1: An overview of chimeric antigen receptor (CAR) structure. Source: Miliotou and Papadopoulou, 2018, Current Pharmaceutical Biotechnology (Vol. 19)
Stages in CAR T-Cell Therapy
There are a series of steps to get the CAR T cell Therapy. These steps are as follows:
- Leukapheresis: In this process, the blood is withdrawn and the autologous T-cells are separated out from the rest of the blood. Collected T-cells are further processed to activate and engineer it specific to tumor.
- Activation of T-cells: T-cell are activated using anti-CD3 antibody and anti-CD28 antibody (for co-stimulatory) signal.
- CAR T cell engineering: In this process, the T-cells are modified to expressed chimer antigen receptors (CARs) on their surface. CARs help the modified T-cells to recognize the tumor cells and mount the immune response.
- Expansion: The engineered T-cells are further expanded and made ready for adoptive transfer.
- Infusion of CAR T cells: At this stage, the CAR T cells are evaluated and optimized to patient conditions like T cell dose, frequency of infusions. Other factors like tumor microenvironment, antigen expression in tumors and safety issues are also considered before it is given to the patient.
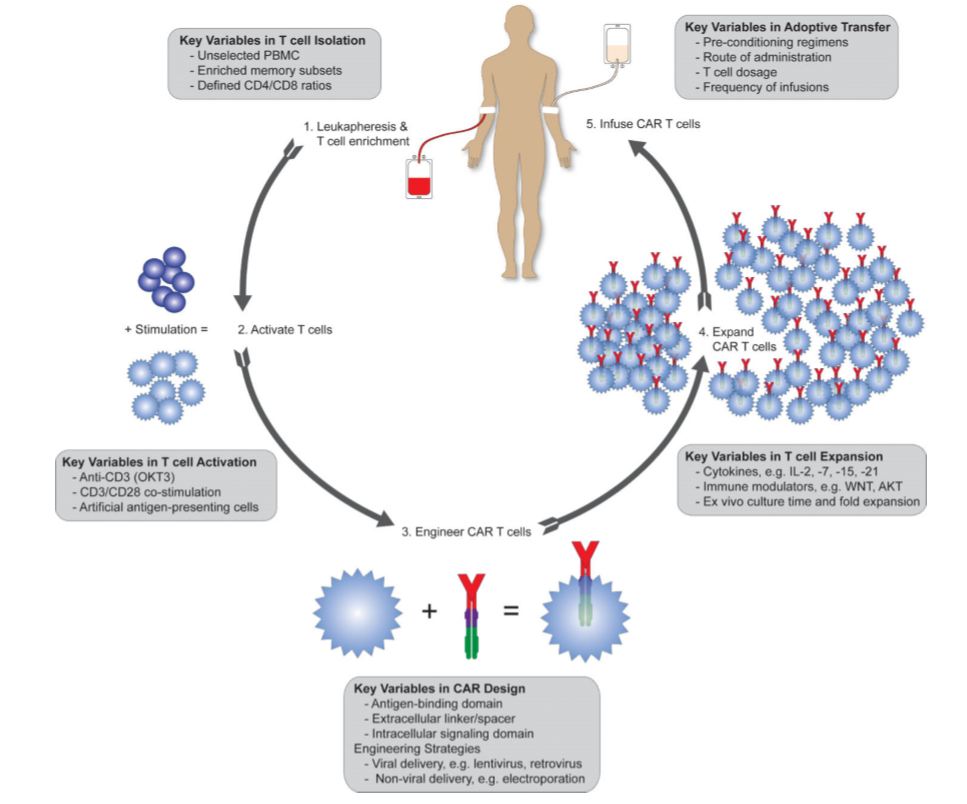
Figure 2: Different Stages of CAR T-cell Therapy. Source: Priceman et al, 2015, Current Opinion on Oncology
Tumor Killing Mechanism of CAR T-cell
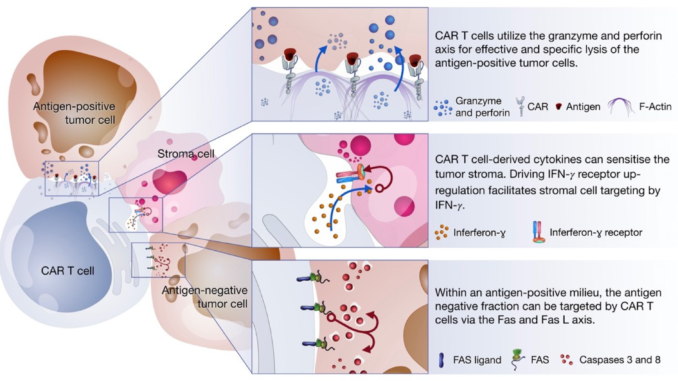
CAR T-cells are designed to eliminate tumor cells by recognizing the antigen expressed on tumor cells without being processed using MHC molecule. T-cells (irrespective of CD8+ or CD4+) can recognize the antigens on cancer cells and kill the cancer cells via either release the perforin and granzyme or by initiating apoptosis via receptor signaling via Fas/Fas-ligand (Fas-L) or TNF/TNF-receptor (TNF-R). These can also act via production of cytokine which further intensifies immune response to kill the tumor cells via downstream processes.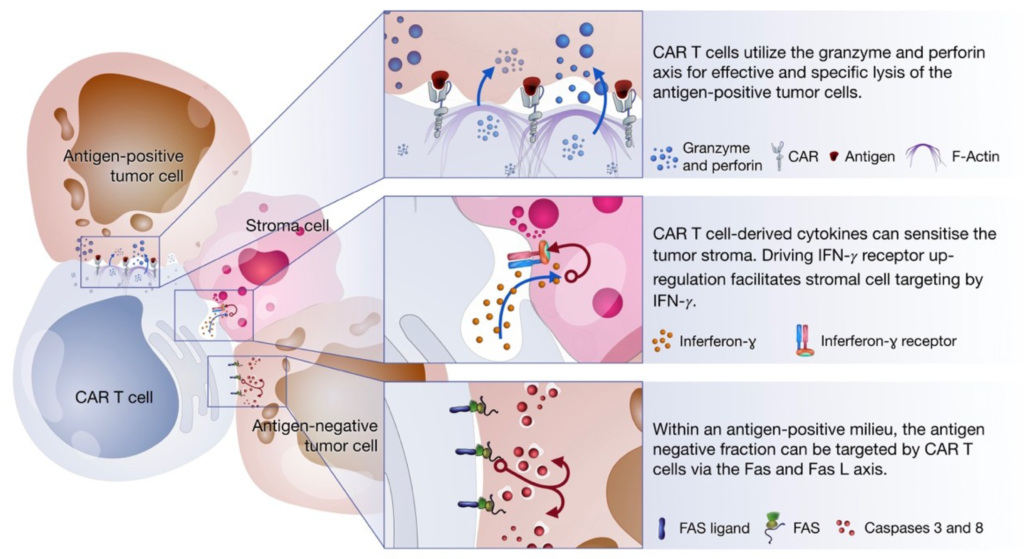
Figure 3: Killing Mechanisms of chimeric antigen receptor (CAR). Source: Miliotou and Papadopoulou, 2018, Current Pharmaceutical Biotechnology (Vol. 19)
Current FDA Approved CAR T-cell Therapies
Tisagenlecleucel (KymriahTM), Axicabtagene ciloleucel (YescartaTM) and Tocilizumab (Actemra®) are the three Food and Drug Administration (FDA) CAR T-cell therapy so far.
Tisagenlecleucel is approved for treatment Acute Lymphobasltic Leukemia (ALL) and refractory large B-cell lymphoma for patients under 25 years age. Tocilizumab is approved for treatment of cytokine release syndrome (CRS). Axicabtagene ciloleucel is approved for refractory large B-cell lymphoma after two or more lines of systemic therapy.
Calibr (a R&D division of Scripps Research Institute received the FDA approval for IND (Investigational new drug) refractory B-cell lymphomas.
Side Effects of CAR-T cell therapy
The possible side effect of CAR T-cell therapy are cytokine release syndrome and neurologic difficulties. In cytokine release syndrome, CAR T-cell therapy could trigger the release of multiple cytokines leading to the inflammatory condition with high fever, flue like symptoms, chills, low blood pressure including difficulty in breathing. Neurologic side effects can include confusion, difficulty in understating speaking and understanding.
References:
- Benmebarek, M. R., Karches, C. H., Cadilha, B. L., Lesch, S., Endres, S., & Kobold, S. (2019). Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. International journal of molecular sciences, 20(6), 1283. https://doi.org/10.3390/ijms20061283
- Miliotou AN, Papadopoulou LC. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol. 2018;19(1):5-18. doi:10.2174/1389201019666180418095526
- Priceman, S. J., Forman, S. J., & Brown, C. E. (2015). Smart CARs engineered for cancer immunotherapy. Current opinion in oncology, 27(6), 466–474. https://doi.org/10.1097/CCO.0000000000000232
- Newick K, O’Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139-152. doi:10.1146/annurev-med-062315-120245
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric antigen receptors modified T-cells for cancer therapy. J. Natl. Cancer Inst., 2016, 108(7), pii, djv439.
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Experim. Med., 2017, 214(4), 895-904
- Fedorov, V.D.; Themeli, M.; Sadelain, M. PD-1- and CTLA-4based inhibitory chimeric antigen receptors (iCARs) divert offtarget immunotherapy responses. Sci. Translatl. Med., 2013, 5(215), 215ra172
- https://www.lls.org/treatment/types-of-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy#FDA Approved Treatments