What are echinocandins?
- Echinocandins are a new and unique class of antifungal agents. They contain a core composed of cyclic hexapeptide linked to lipid side chains responsible for their antifungal activity.
- Echinocandin B is first echinocandin to be discovered as antifungal agent in 1970s. However, it is not used due to risk of hemolysis. Caspofungin acetate was first echinocandin to get marketing approval. Micafungin and anidulafungin are other approved echinocandins.
- Caspofungin was approved for use in United States in 2001, micafungin in 2005 and anidulafungin in 2006.
Indications of echincandins
- Caspofungin is approved for treatment of candidemia (presence of yeast or fungi in bloodstream), candida esophagitis (infection of esophagus by albicans), salvage therapy of Aspergillus infections and for febrile neutropenia.
- Micafungin is used for candida esophagitis and antifungal prophylaxis for hemopoietic stem cell transplant recipients.
- Anidulafungin is approved for treating candidemia and candida esophagitis.
Anti-fungal activity of echinocandins
- They possess fungicidal activity against Candida and fungistatic Aspergillus species including those species resistant to azoles. They may also be effective against other fungi such as Histoplasma capsulatum, C. immitis . Micofungin exhibit more potent activity against mycelial forms of H. capsulatum, C. immitis and B. dermatitidis.
- The cell wall of C. neoformans consist mainly of α- (1,3)- or α- (1,6)- glucan so they are resistant to echinocandins.
Mechanism of action of echinocandins
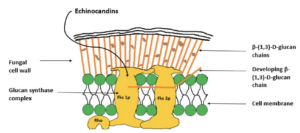
Figure- Mechanism of action of echinocandins (source- Patil et al, 2017)
- Echinocandins are non-competitive inhibitor of the enzyme β- (1,3)- glucan synthase which catalyzes polymerization of UDP-glucose into β- (1,3)-glucan, a structural component of fungal wall. Thus, they inhibit synthesis of β- (1,3)-glucan leading to loss of cell integrity and rigidity and ballooning of cellular content from weakened cell wall resulting in cell lysis.
- Glucan synthase is not found in mammalian cells, so echinocandins doesn’t posses serious effect on mammalian cells.
Development of resistance
- Organisms like C. neoformans which don’t depend on β- (1,3)-glucan are less susceptible to echinocandins. They have little to no in-vitro activity against B. dermatitidis, Fusarium sp, Sporothrix schenckii and other Zygomycetes.
- Echinocandin resistance is rare among Candida. However, different cases have shown some resistance in C. albicans, C. glabrata, C. tropicalis and C. parapsilosis. Resistance may be due to alteration in glucan synthase, overexpression of efflux pump, mutation in FKS genes and strengthening of cell wall by increased chitin synthesis.
- Prolonged or repeated exposure to echinocandins is main factor responsible for development of resistance among Candida spp.
Pharmacokinetics of echnicandins
- They have low oral bioavailability and are available as IV infusion. They bind with plasma proteins and have low volume of distribution. The volume of distribution of anidulafungin is greater than of micafungin.
- They are metabolized mainly in liver. Caspofungin is metabolized by hydrolysis and N-acetylation. Dosage adjustment is recommended in hepatic patient for caspofungin.
- Micafungin have two metabolites which possess anti-fungal activity. These products are excreted slowly over may days, mainly in bile.
- None of them are excreted by kidney in great extent so dose adjustment is not required in renal patients.
- They don’t cross BBB (Blood Brain Barrier) and concentration in brain tissues are relatively low.
Adverse Effects of echinocandins
- Adverse events are mild and infrequent. The most common complications associated with infusion of all three echinocandins include facial flushing, swelling, rash, fever, thrombophlebitis and hypotension. Elevated level of histamine can be seen.
- Caspofungin may cause face edema, respiratory symptoms including wheezing and bronchoconstriction. Micafungin is associated with nausea, vomiting, bilirubinemia and increased liver function test level.
- The most common adverse events associated with anidulafungin are headache, nausea, dizziness and elevated alanine transaminase level.
Drug Interaction
- As they are poor substrate of cytochrome P 450 enzymes, fewer drug interactions are observed. Concurrent administration with cyclosporine may cause mild, reversible elevation in hepatic transaminases. Precise mechanism behind this interaction is unknown.
- Co-administration of inducer or mixed inducer/inhibitor of CYP 450 enzymes like phenytoin, dexamethasone, rifampin, nevirapine, carbamazepine and efavirenz can reduce concentration of caspofungin. Although co-administration is not contraindicated, monitoring of caspofungin level or dose escalation may be considered.
Contraindication
- They are embryotoxic so are avoided during pregnancy.
References
- https://uspharmacist.com/article/the-fungus-among-us-an-antifungal-review
- https://www.ncbi.nlm.nih.gov/books/NBK548299/
- https://experts.umn.edu/en/publications/echinocandin-antifungals-review-and-update
- antimicrobe.org/d35.asp
- Yamashitaab C, Takesueac Y, Matsumotoad K, Ikegameae K, YukiEnokid Y, Uchinoaf M et al. Echinocandins versus non-echinocandins for empirical antifungal therapy in patients with hematological disease with febrile neutropenia: A systematic review and meta-analysis. Journal of Infection and Chemotherapy. 2020; 26(6): 596-603.
- Bussche HLV, Loo DAV. A Clinical Review of Echinocandins in Pediatric Patients. Annals of Pharmacotherapy. 2010; 44(1).
- Mroczynska M, Dabrowska Ab. Review on Current Status of Echinocandins Use. Antibiotics (Basel). 2020; 9(5): 227.
- Denning DW. Echinocandin antifungal drugs. The Lancet. 2003; 362: 1142-1151.
- Patil A, Majumdar S. Echinocandins in antifungal Pharmacotherapy. Journal of Pharmacy and Pharmacology. 2017; 69: 1635-1660.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.