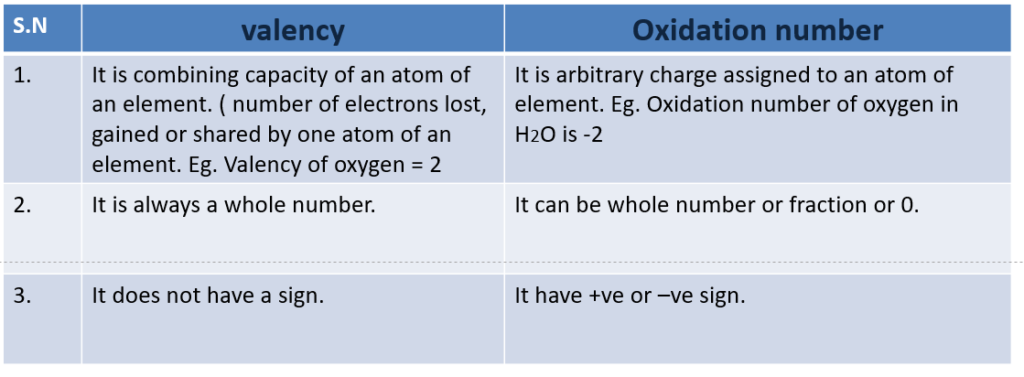
Classical concept of oxidation and reduction
Oxidation:
Oxidation is the chemical process which involves addition of oxygen or any other electronegative species(element) or removal of hydrogen or any other electropositive species.
for example;
I. Addition of oxygen

Here, oxygen is added to Carbon to form CO2. Hence, C is oxidized to CO2. Conversion of C into CO2 is oxidation.
Note: every atom or molecule have zero oxidation state, when they are in free state.
When electro negative (positive) species combined with other element they gain(lose) electron and become –vely (+vely) charged, which is termed as oxidation state of that species.
II. Addition of electronegative species

Here is oxidation of Fe due to addition of electronegative species (Cl).
III. Removal of hydrogen

Here hydrogen is removed from H2S to form S, where S is oxidized and the reaction is oxidation reaction
IV. Removal of electropositive species

Here potassium (electropositive element) is removed from KI to form I2, where I2 is oxidized and the reaction is oxidation reaction.
Reduction
Reduction is the chemical process which involves the addition of hydrogen or any other electropositive species or removal of oxygen or any electronegative species.
For example;
I. Removal of oxygen

Here oxygen is removed from ZnO to form Zn, where ZnO is reduced to Zn and the reaction is reduction reaction.
II. Removal of electronegative species

Here, Cl is removed from FeCl3 to FeCl2. Conversion of FeCl3 to FeCl2 is reduction.
III. Addition of hydrogen

Here, hydrogen is added to Cl2 to form HCl. Hence Cl2 is reduced to HCl.
IV. Addition of electropositive species.

Here, potassium is added to Cl2 to form KCl. Hence Cl2 is reduced to KCl.
Modern or electronic concept of oxidation and reduction
( It is based on the concept of loss or gain of electron)
Oxidation:
According to this concept, oxidation is the chemical process which involves loss of electrons. This is also called de-electronation process.
For example:

(Increase in +ve charge =loss of electron = oxidation= de – electronation process= decrease in –ve charge)
Reduction :
According to this concept, reduction is the chemical process which involves gain of electrons. This is also called electronation process.
For example:

(Incease in –ve charge= gain of electron= reduction= electronation process = decrease in positive charge).
Oxidation number or oxidation state:
Oxidation number may be defined as the charge which appears to be associated with the atom on removing all other atoms as ions.
Rules of determining oxidation number
•Oxidation number of an element in elementary (free) state is zero. •Algebraic sum of oxidation numbers of all elements in a compound is equal to zero.
•Algebraic sum of oxidation number of all elements in an ion is equal to the charge on the ion. Eg SO4—
•Oxidation number of alkali metal (Li, Na, K, Rb, Cs, and Fr,) is +1 in all compounds.
•Oxidation number of alkaline earth metal (Be, Mg, Ca, Sr, Ba, and Ra) is +2 in all compounds.
•The oxidation number of hydrogen in compound is +1, except in metallic hydrides. In metallic hydrides oxidation number of hydrogen is -1. for eg. NaH, CaH2
•Oxidation number of oxygen is -2 in all compounds except in peroxides, superoxides and in OF2.
Example : in peroxide O.N of oxygen is -1 (H2O2) H-O-O-H
in superoxide O.N of oxygen is -1/2 (KO2) +1 + 2x =0
in OF2 O.N of oxygen is +2
•Oxidation number of metals in alloy is zero. Eg. Na, Hg
Calculate the oxidation state of Fe in Fe3O4.
This is the mixed oxide of Fe( ferroso ferric oxide : FeO and Fe2O3 ). There are two types of Fe,
FeO X+(-2)=0
Fe2O3 2X+(-2*3)=0
Difference between oxidation number and valency