- Ondansetron is one of the most commonly used anti-emetic agent. It belongs to class of first generation 5-HT3 receptor antagonist and is also in the World Health Organization’s List of Essential Medicines. It was developed by Glaxosmithkline in 1980s and got its FDA approval in 1991.
- Other 5-HT3 receptor antagonist approved for use in nausea and vomiting include granisetron, dolasetron and palonosetron.
Indications of Ondansetron
- Used for nausea and vomiting caused by chemotherapy, radiotherapy, or anesthesia.
- Used to treat post-operative nausea and vomiting.
- In nausea and vomiting evoked by drug poisoning and overdose.
- Also used in irritable bowel syndrome, vertigo, chronic refractory diarrhea, diarrhea associated with diabetes, alcohol withdrawal, cerebellar tremor, and Parkinson’s disease treatment related psychosis.
Mechanism of action of Ondansetron
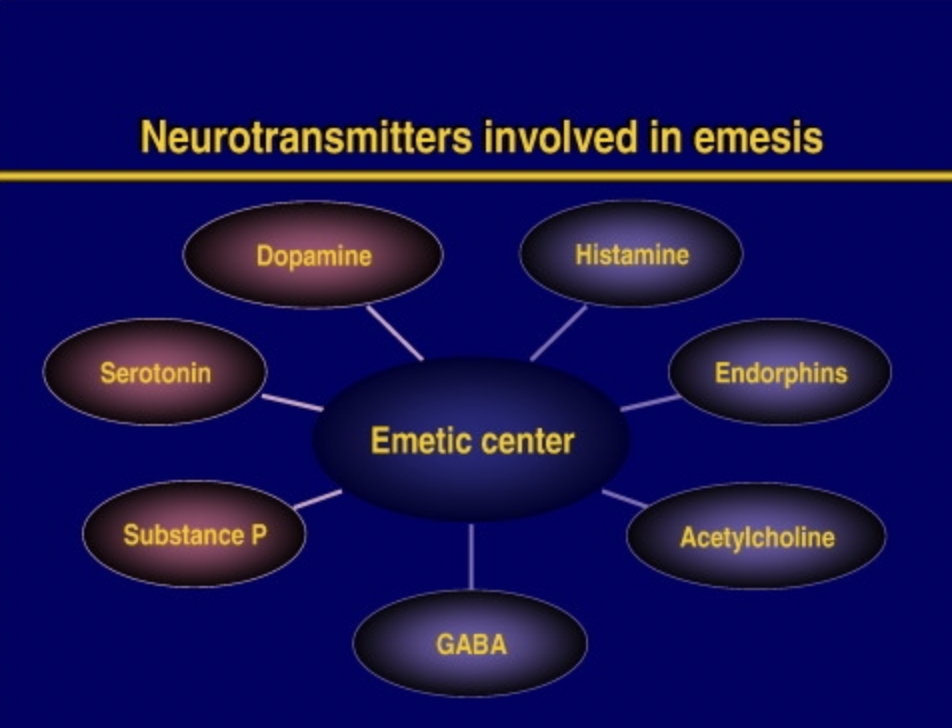
Figure- Neurotransmitters involved in emesis (source- Navari RM etal, 2015)
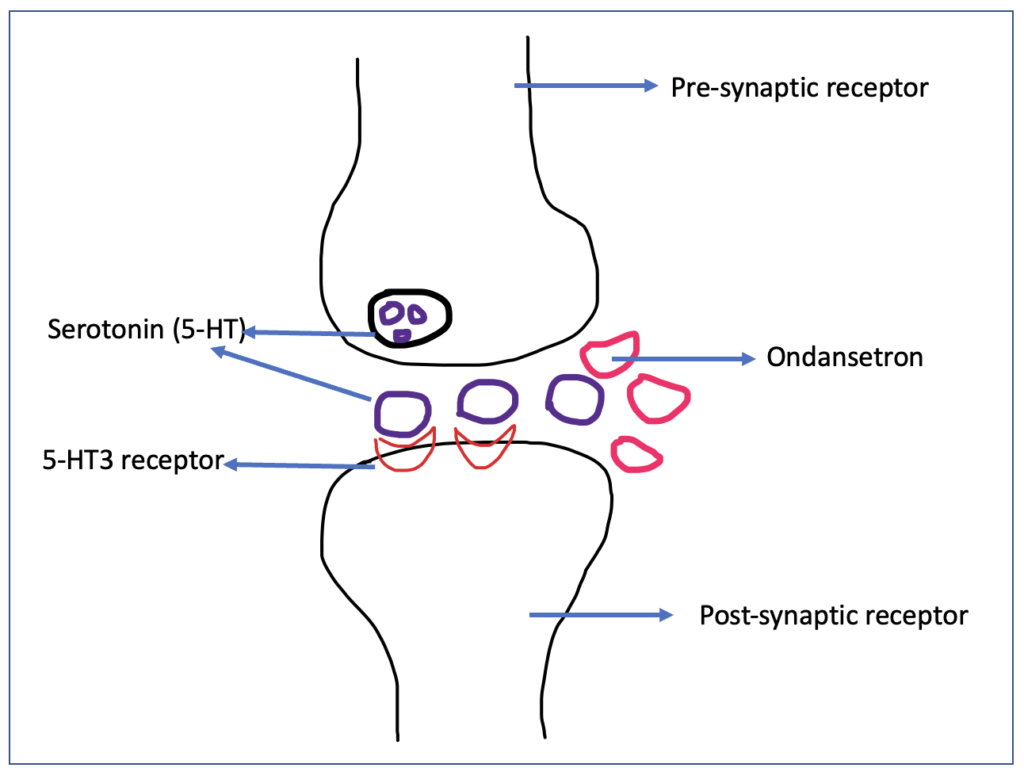
Figure- Mechanism of action of Ondansetron
- It is a selective antagonist of 5-HT3 receptor (5- hydroxytryptamine-3 receptor).
- 5-HT3 receptors are ligand gated ion channel which are present in both the peripheral and central nervous system. Serotonin or 5- hydroxy tryptamine is the agonist acting on 5-HT3 receptor. These receptors play role in different function including cognition, emesis, and anxiety. 5-HT3 receptors are present in area postrema which contain CTZ (chemoreceptor trigger zone) and CTZ play role in mediating nausea and vomiting.
- 5-HT3 receptor are also present in vagus nerve terminals (peripheral nervous system) which play role in sensing nausea and vomiting triggered within GI system such as by stomach irritants.
- Ondansetron acts on 5-HT3 receptor present both in CNS and PNS and prevent nausea and vomiting.
Pharmacokinetics of Ondansetron
- It can be administered via oral, IV or IM route. When administered via oral route, it is given 30 minutes before chemotherapy, 1 hour before anesthetic agents and 1-2 hours before radiotherapy. Oral administration with food or antacid doesn’t have any effect on its absorption.
- When given through oral route, it undergoes first pass metabolism and hence its oral bioavailability is only 60%. It reaches to its peak plasma concentration after around 1.5 hours of oral administration.
- Around 75% of administered drug binds to plasma protein. Its metabolism takes place in liver by cytochrome P450 enzymes like CYP1A2, CYP2D6 and CYP3A4. Excretion takes place via urine and feces. Dose may depend on route of administration
Adverse effects
- Some of its common side effects include headache, dry mouth, constipation, fatigue. Side effects which are less common include drowsiness, sedation, transient increase in liver function test. It can cause dose dependent QTc interval elongation. So elderly patients taking ondansetron should be monitored potassium and magnesium level and ECG monitoring.
- Compared to other route of administration, IV route has greater risks. So, while using IV route, single dose greater than 16 mg are not recommended.
Contraindications
- Contraindicated in patients with hypersensitivity or allergic to ondansetron.
Drug Interactions
- Ondansetron may enhance hypotensive effect of apomorphine (apomorphine is used to treat off episodes in patients with Parkinson’s disease) when used together and cause profound hypotension and loss of consciousness. So, ondansetron is not used with apomorphine.
- It is pregnancy risk-factor category B drug and used only when pregnancy related nausea and vomiting cannot be treated by other anti-emetics like diphenhydramine, meclizine, promethazine etc.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2664614/
- https://www.ncbi.nlm.nih.gov/books/NBK499839/
- https://www.sciencedirect.com/science/article/abs/pii/S0002962915355919
- https://www.researchgate.net/publication/11869114_Ondansetron_A_Selective_5-HT3_Receptor_Antagonist_and_Its_Applications_in_CNS-Related_Disorders
- https://go.drugbank.com/drugs/DB00904
- https://www.sciencedirect.com/science/article/pii/S0005273615000991
- A textbook of Clinical Pharmacology and Therapeutics. 5th edition.
- Goodman and Gillman’s Manual of Pharmacology and Therapeutics.
- Lippincott’s Illustrated Reviews Pharmacology. 6th edition.
- Pharmacology and Pharmacotherapeutics. 24th edition.