- Lomitapide is lipid lowering agent which reduces blood level of ‘bad’ cholesterol. It is recently approved for treatment of homozygous familial hypercholesterolemia.
- Lomitapide is an agent with novel mechanism of action.
Indications of Lomitapide
- Approved by FDA in 2012 for treatment of homozygous familial hypercholesterolemia- a rare form of hypercholesterolemia which can lead to premature atherosclerotic disease. Homozygous familial hypercholesterolemia is characterized by very high plasma level of total and low-density lipoprotein cholesterol level, cutaneous xanthomas and atherosclerotic coronary artery disease from childhood.
- It is used in combination with low fat diet and other lipid lowering agents and plasma lipid apheresis.
Mechanism of action of Lomitapide
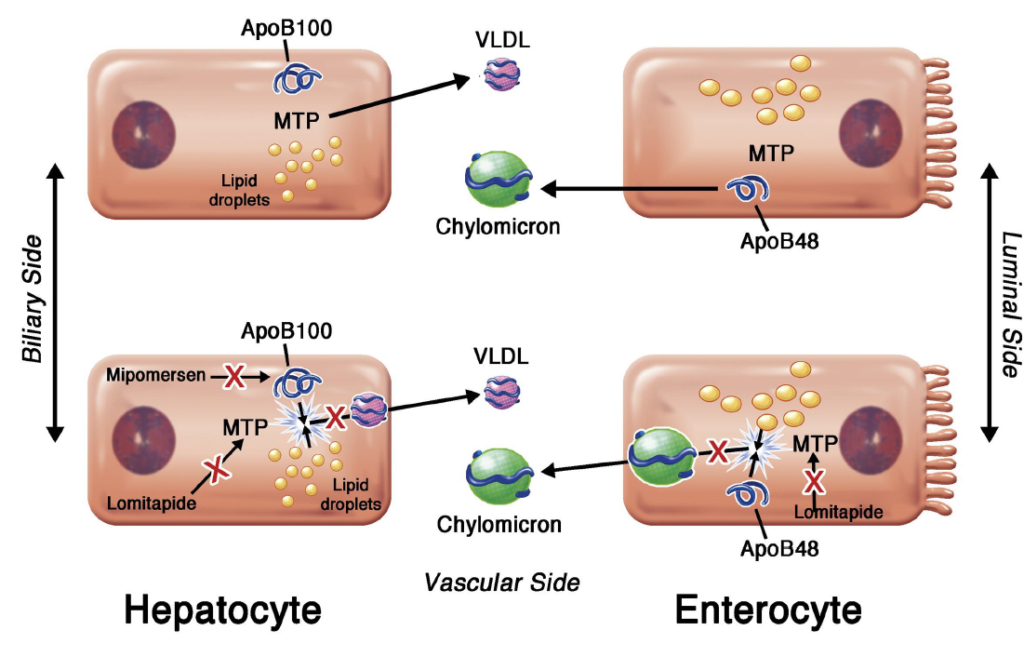
Figure- Inhibition of VLDL and chylomicron synthesis by lomitapide (Source- Fazio et al, 2014)
- It is an inhibitor of MTP (Microsomal triglyceride transfer protein)- an enzyme present in endoplasmic reticulum of hepatocytes and enterocytes. This enzyme transfer triglyceride to nascent apoB and helps in synthesis of VLDL (Very Low-Density Lipoprotein) in liver and chylomicron in intestine.
- Lomitapide directly binds to MTP and inhibit transfer of triglyceride to apoprotein B 48 and apoprotein B 100. Hence, it inhibits synthesis of chylomicron and VLDL.
- It lowers LDL by 40-50 % from pretreatment alone or in combination. The reduction in VLDL is about 30-50%.
Pharmacokinetics of Lomitapide
- It is administered as oral capsule at least 2 hours after evening meal. It is prescribed under a specific program REMS (Risk Evaluation and Mitigation Strategy) approved by FDA.
- Lomitapide is metabolized in liver by CYP3A4 enzymes. Around 50% of administered dose is excreted in urine and 33-35 % in feces.
- Its half-life is about 39.7 hours.
Dosage
- The initial dose is 5mg/day which can be increased at 4-week interval to maximum of 60 mg/day. It should be taken with water and without food at least 2 hours after evening meal.
- Along with this medicine, patient should take supplement like vitamin E, linoleic acid, alpha-linoleic acid, eicosapentaenoic acid and docosahexaenoic acid. These supplements helps to reduce development of fat soluble nutrition deficiency. Dosage adjustment is needed in patients if level of aspartate or alanine amino transferase is three times the normal level or higher.
Adverse effects
- It has black box warning of hepatotoxicity as it causes fat accumulation in liver.
- As it causes inhibition of fat absorption, significant GI related adverse effects like nausea, dyspepsia, vomiting and diarrhoea are common. These effects are seen in around 93% of patients.
Drug Interactions
- As it is metabolized by CYP3A4, drug interaction is common with inhibitors of CYP3A4. It strongly interacts with drugs like clarithromycin, ketoconazole, itraconazole and some protease inhibitors. It moderately interacts with erythromycin, verapamil and fluconazole.
- When used in combination with weak CYP3A4 inhibitor like oral contraceptives and atorvastatin, its dose should not exceed more than 30 mg/day.
- When used in combination with warfarin, it may increase value of INR (International Normalized Ratio). So, patient receiving this combination should be regularly monitored for INR.
Contraindication
- Its use with strong and moderate CYP3A4 inhibitors are contraindicated.
- It is contraindicated in patients with liver disease and patients with sustained abnormal liver function test.
- Contraindicated in pregnancy.
References
- Davis KA, Miyares MA. Lomitapide: A novel agent for the treatment of homozygous familial hypercholesterolemia. American Journal of Health-System Pharmacy. 2014; 71(12): 1001-1008.
- Dave etal. Lomitapide and Mipomersen: Novel Lipid-Lowering Agents for the Management of Familial Hypercholesterolemia. The Journal of Cardiovascular Nursing. 2014; 29(5): E7-E12.
- Pharmacology Update: Lomitapide Capsules (Juxtapid) | 2013-02-28 | AHC Media: Continuing Medical Education Publishing (reliasmedia.com)