- Lead is one of the oldest known and most widely studied environmental and occupational toxins. Because of unique properties of lead, like softness, high malleability, ductility, low melting point and resistance to corrosion, it is used widely in different industries like automobiles, paint, ceramics, plastics, etc. This has led to a manifold rise in the occurrence of free lead in biological systems and the inert environment.
- The primary sources of environmental exposure to lead are leaded paint and drinking water. Poisoning may also occur from petrol containing lead and lead projectiles, lead containing pipes or lead-based solder in water supply systems, battery recycling, grids and bearings, etc.
Mechanism of action of Lead Poisoning
- Toxicity of lead is due to its affinity for cell membrane and mitochondria. It impairs the activity of calcium dependent intracellular messengers and of brain protein kinases. Loss of red cells is due to medullary inhibition of erythropoiesis. There is also a direct action of lead on the red cell membranes and their enzymes that increases their fragility and shortens their life span.
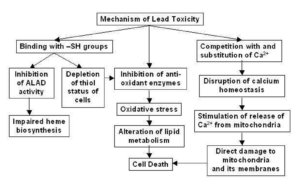
Figure- Possible Mechanism of Lead toxicity (Source- Flora SJS et al, 2007)
Oxidative stress
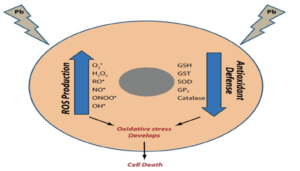
Figure- Effect of antioxidants on lead induced oxidative stress (Source- Sani AH et al, 2021)
- It is major mechanism of lead induced toxicity which occurs due to simultaneous operation of two different pathways. The first is generation of ROS (Reactive Oxygen Species) like hydroperoxide, singlet oxygen and hydrogen peroxide and second is depletion of antioxidant reserves. Lead shows electron sharing capability that results in the formation of covalent attachments with the sulfhydryl groups present in antioxidant enzymes, which are the most susceptible targets for lead, and which eventually get inactivated. Lead inactivates glutathione, most important antioxidant found in cells by binding to sulfhydryl groups present in it.
- Similarly, lead inactivates enzymes like δ-amino levulinic acid dehydratase (ALAD), glutathione reductase (GR), glutathione peroxidase (GPX) and glutathione-S-transferase, which further depresses the glutathione levels. A few other antioxidant enzymes that are rendered inactive by lead include super oxide dismutase (SOD) and catalase (CAT).
- Lead also causes hemoglobin oxidation, which directly causes RBC hemolysis. This occurs due to inhibition of ALAD (Aminolevulinate Dehydratase), which results in an increased concentration of substrate ALA (Aminolevulinic acid) in both blood and urine. These elevated ALA levels generate hydrogen peroxide and superoxide radical and interact with oxyhemoglobin, resulting in the generation of hydroxyl radicals. Progression of all the above-mentioned mechanisms makes the cell extremely vulnerable to oxidative stress and may lead to cell death.
Ionic mechanism of lead toxicity
- It is due to its ability to substitute other bivalent cations like Ca2+, Mg2+, Fe2+ and monovalent cations like Na+ which affect various fundamental biological processes of the body like intra and intercellular signaling, cell adhesion, protein folding and maturation, apoptosis, ionic transportation, enzyme regulation, release of neurotransmitters, etc.
- The lead replaces calcium ions and becomes competent to cross the blood-brain barrier (BBB). After crossing the BBB, lead accumulates in astroglial cells (containing lead binding proteins). Toxic effects of lead are more dangerous in the developing nervous system comprising immature astroglial cells that lack lead binding proteins. Lead easily damages the immature astroglial cells and obstructs the formation of myelin sheath, both factors involved in the development of BBB. At a neuronal level, exposure to lead alters the release of neurotransmitter from presynaptic nerve endings. Even in small concentration, lead can replace calcium and affect key neurotransmitters like protein kinase C, which regulates long term neural excitation and memory storage.
- It also affects the sodium ion concentration, which is responsible for vital biological activities like generation of action potentials, uptake of neurotransmitters (choline, dopamine and GABA) and regulation of uptake and retention of calcium by synaptosomes. This interaction between lead and sodium seriously impairs the normal functioning of the sodium dependent processes.
Acute Lead Poisoning
- The chances of acute lead poisoning are rare and may occur due to ingestion of acid-soluble lead compound or inhalation of lead vapor. Metallic taste is produced within mouth. Symptoms like GI irritation, vomiting, muscle cramp, weakness is observed. The vomitus may be milky due to presence of lead chloride and stool may be black due to presence of lead sulphide. An acute hemolytic crisis may occur leading to severe anemia and hemoglobinuria. The kidneys are damaged. Death may occur within 1-2 days.
- A simple blood test can be used for detecting lead poisoning. There is no such level of lead that appears to be necessary or beneficial to the body and no “safe” level of exposure to lead has been found. For children a level of 5 mcg/dL indicate a possibly unsafe level. Children having blood tests at those levels should be tested periodically. A child whose levels become too high, generally 45 mcg/dL or higher should be treated.
- Treatment consist of gastric lavage and use of laxatives like magnesium sulphate to evacuate lead from gut. Chelating agents like DMSA (2,3-dimercapto succinic acid) can be used. According to one research, combined administration of DMSA and antioxidant like NAC (N-acetyl cysteine) could be a more effective treatment protocol for acute lead toxicity, due to its beneficial effect on oxidative injury. Parenteral fluids and vasopressor agents can be used to treat shock, anti-cholinergic like atropine can be used for intestinal colic.
Chronic lead poisoning
The manifestations of chronic lead poisoning are;
- Gastrointestinal: GI syndromes which is more prevalent in adults represent very slow and insidiously developing intoxication. Metallic taste in mouth, anorexia, constipation and abdominal pain are common symptoms. Grayish lead line which appears along the gingival margin is diagnostic feature of importance.

- Haemopoietic System: Lead may impair several steps in synthesis of heme. The step involving the enzyme amino-levulinic acid dehydratase is impaired, leading to increased excretion of this enzyme. Measurement of this metabolite is useful component of screening. Other symptoms are microcytic hypochromic anemia and jaundice.
- CNS system: The symptoms are more prevalent in children and are termed as lead encephalopathy. Adults rarely have such symptoms unless there is massive exposure to lead vapors or organic form of lead. Common symptoms include headache, increased irritation, tremors, delirium with repetitive tonic-clonic convulsions followed by lethargy and coma. Visual disturbance is also seen. Lead encephalopathy has a mortality of 25% and nearly 1/4th of the survivors develop permanent mental deficiency. Lead poisoning may produce progressive mental deterioration in children, followed by a steady loss of motor skills and speech. It may cause severe hyperkinetic and aggressive behavior disorders and a poorly controllable convulsive disorder.
- Renal system: Renal effects are less obvious than CNS and haemopoietic effects. It may cause tubular damage, hyperuricemia and renal failure. It had been seen that persons who have survived acute lead poisoning in childhood may have increased incidence of chronic nephropathy in adulthood.
Diagnosis of lead poisoning
- At present, the blood level concentration of lead is the only method used for diagnosis of acutely elevated lead absorption. The blood lead level rises rapidly within hours after an acute exposure and remains elevated for several weeks. The interpreted unexposed or normal level of lead in blood is ≤ 9 µg/dL. Till now a precise blood lead level below which symptoms never occur or a blood lead level at which symptoms are always reported hasn’t been determined. Individual susceptibility should always be recognized.
- The normal urinary concentration of lead is generally <80 mg/L (0.4 mM) in adults. In case of lead poisoning concentrations of lead in urine may reach 150–300 mg/L (0.7–1.4 mM). However, in persons with chronic lead nephropathy or other forms of renal disorders, urinary excretion of lead may be within the normal range, even though blood lead concentrations are significantly elevated.
- There are high chances of missing diagnosis of lead poisoning easily in the absence of a positive history of abnormal exposure to lead, because most of the signs and symptoms of lead poisoning are seen in other diseases.
Treatment
- The first step should be removal of individual from exposure. Whether removal from exposure is enough, or chelation therapy should be administered depends on the blood lead concentration, the severity of clinical symptoms, the biochemical and hematologic abnormalities. There are no specific blood lead concentrations that can be used to determine when treatment with a chelating agent is indicated. However, such a level is usually well above 80 μg per dL which is also the level associated with more severe symptoms.
- Chelating agents used are succimer, dimercaprol, d-penicillamine and calcium disodium edetate. The initial dose of calcium disodium edetate is 30–50 mg/kg/day in 2 divided doses either by deep IM injection or slow IV infusion for up to 5 consecutive days. Each course of therapy should not exceed a total dose of 500 mg/kg. Urine output must be monitored.
- Dimercaprol is given intramuscularly at a dose of 4 mg/kg every 4 hours for 48 hours, then every 6 hours for 48 hours, and finally, every 6–12 hours for an additional 7 days. Penicillamine is effective orally and given at a dosage of 250 mg 4 times daily for 5 days. Its dose should not exceed 40 mg/kg/day. Succimer is the first orally active lead chelator available for children, given every 8 hours (10 mg/kg) for 5 days and then every 12 hours for an additional 2 weeks.
- The combination of dimercaprol and calcium disodium edetate or calcium disodium edetate and succimer is more effective than is either chelator alone.
Prevention
- Public awareness about environmental hazards of lead should be increased through education, documentation and communication. Actions should be taken to prevent overexposure. Primary prevention can be achieved using engineering controls like substitution of lead with less hazardous material, ventilation through local exhaust system, personal protective equipment and good work practices like personal hygiene practices, housekeeping activities to remove lead dust accumulation etc.
References
- A. Gidlow. Lead toxicity. Occupational Medicine. 54 (2): 76-81.
- Joseph P. Bressler, Gary W. Goldstein. Mechanisms of lead neurotoxicity. Biochemical Pharmacology. 41(4): 479-484.
- Gagan Flora, Deepesh Gupta, Archana Tiwari. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012; 5(2): 47–58.
- Flora SJS et al. Arsenic and Lead induced free radical generation and their reversibility following chelation. Cellular and Molecular Biology. 53(1): 26-47.
- Manish Pande, Ashish Mehta, Bhagwat P Pant, Swaran JS Flora. Combined administration of a chelating agent and an antioxidant in the prevention and treatment of acute lead intoxication in rats. Environmental Toxicology and Pharmacology. 9(4): 173-184.
- A. Gover. Lead toxicity: a problem in environment pathology. Am J Pathol. 64(1): 167-182.
- Menezes, H.S. D’souza, T. Venkatesh. Chronic lead poisoning in an adult battery worker. Occupational Medicine. 53(7): 1 October 2003: 476–478.
- Kevin C. Staudinger, Victor S. Roth. Occupational Lead Poisoning. Am Fam Physician.1998 Feb 15;57(4):719-726.
- Pharmacology and Pharmacotherapeutics book, R.S. Satoskar. Page no- 1097-1099.
- Goodman and GILLMAN’S Manual of Pharmacology and Therapeutics. Page no- 1130-1134.
- https://www.ncbi.nlm.nih.gov/books/NBK541097/