Heavy Metal Antagonists
Heavy metals which have widespread environmental distribution and originate from natural and anthropogenic sources are common environmental pollutants. All heavy metals are cumulative and potentially toxic which can cause damage to organs like liver, kidney, gut, brain. The toxic effect of heavy metals is due to their ability to form complexes with important biological radical like sulfhydryl, the hydroxyl, the carboxyl, the imidazole etc.
Heavy metal antagonists are drugs which combine with metallic ions to produce non-toxic and easily water-soluble complexes. In this process, the relatively stable non-ionized ring complex formed between metal and compound is known as chelate. The process is known as chelation and the compounds are termed as chelating agents. Effectiveness of chelating agent for treatment of heavy metal poisoning depends on certain factors:
- The relative affinity of chelator for the heavy metals compared to essential body metals
- The distribution of chelator in the body as compared to distribution of heavy metals
- Capacity of chelator to remove the metal from the body once chelated.
1)Dimercaprol
British anti-Lewisite (BAL) or dimercaprol was developed during world war-II to minimize the risk to injury or death from Lewisite, a very potent arsenic based warfare agent. It is an oily fluid with pungent odor.
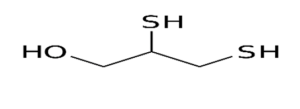
Figure: Dimercaprol
Mechanism of action
Its pharmacological action is due to formation of stable five membered ring complex between its -SH group and metals thereby neutralizing its toxicity and promoting its elimination. Dissociation of dimercaprol- heavy metal complex may occur in-vivo sometimes, which increases chance of release of metal in active form. So, the dosage regimen is designed in such a way that free drug is always present in the body to bind with free metal released from dissociation. Dimercaprol is much more effective when given sooner after exposure as it is better at preventing enzyme inhibition rather than reactivating enzyme function.
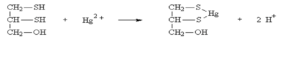
Figure- Mechanism of action of dimercaprol by forming complex
Pharmacokinetics
It is given by deep IM injection as a 100 mg/ml solution in peanut oil, thus should not be used in patients allergic to peanuts. Peak plasma concentration occurs within 30 to 60 minutes. Dimercaprol gets distributed in all tissues with more concentration in liver, kidneys, brain and small intestines. It undergoes hepatic metabolism. It has a short half-life and is excreted as inactive metabolites or metal complex.
Toxicity
The administration of dimercaprol produce side-effects like nausea, vomiting, burning sensation in mouth, penis and eyes, lacrimation, salivation, tingling in hands, muscle pain, restlessness, chest tightness etc. Fever occurs in 30 % of children. Injection of BAL is painful which can cause sterile abscesses and drug fever.
Contraindication
It is contraindicated in hepatic diseases and in iron poisoning as it forms toxic complex with iron. In patients with glucose-6-phosphate deficiency, it may cause hemolytic anemia. It is contraindicated in patients allergic to peanuts.
Uses
- Currently dimercaprol is FDA- approved in treating acute poisoning due to arsenic, mercury, gold and lead.
In severe arsenic and gold poisoning, 3mg/kg is given deep IM at 4 hourly intervals for the first 2 days, at 6 hourly intervals on the third day, and twice daily for the next 10 days or until the recovery is complete. In gold poisoning, the maintenance dose may have to be administered for as long as 3 months
In mild arsenic or gold poisoning 2.5 mg/kg is given deep IM every 4 hours for 6 doses, then every 6 hours for 4 doses, then every 8 hours for 3 doses, then every 12 hours for 2 doses, followed by once daily for 10 days.
Lead poisoning: Deep IM: 4 mg/kg every 4 hours for 3 days and 2.5 mg/kg for 1 to 4 days; dimercaprol is given in combination with calcium disodium edetate; dimercaprol should be administered first and calcium disodium edetate should be administered at a different site than dimercaprol.
- In acute poisoning of thallium, antimony.
- In Wilson’s disease: Wilson’s disease is a rare inherited disorder causing accumulation of copper in liver, brain and other vital organs. In patients with Wilsons disease allergic to penicillamine, dimercaprol is given IM in the dose of 2.5 mg/kg bid for first two days, followed by 2 days rest and repeated as often as necessary.
2) Penicillamine:
It is a cysteine analog with reduced sulfhydryl group. The D-isomer of penicillamine is prepared by alkaline hydrolysis of benzyl penicillin.
Mechanism of action
Penicillamine forms water soluble complex with copper, mercury, zinc and lead and promotes excretion of these metals through urine.
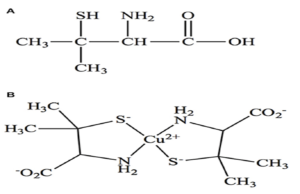
Figure- a) Structure of penicillamine b) Formation of complex by penicillamine
Pharmacokinetics
Penicillamine is well absorbed on oral administration. Peak concentration is obtained within 1-3 hours of administration. It is excreted mostly through urine and less percentage through faeces. Hepatic biotransformation is responsible for degradation of penicillamine.
Toxicity
Common side effects are headache, nausea, vomiting. With long term use penicillamine induces leucopenia, thrombocytopenia, aplastic anemia, sore throat. Renal toxicity is manifested as reversible proteinuria and hematuria, but it may progress to the nephrotic syndrome with membranous glomerulopathy.
Uses
- For treatment of copper, lead and mercury poisoning.
- For treatment of Wilson’s disease:
Penicillamine is administered in the dose of 1-2 g (base) daily, in divided doses, before food. Depending on the urinary levels of copper, the dose may be subsequently increased to 4 to 5 g daily and continued indefinitely. Foods rich in copper should be avoided.
- For treating hereditary avian dystrophy.
- For treating cystinuria.
- In treatment of rheumatoid arthritis: A single daily dose of 125–250 mg usually is used to initiate therapy, with dosage increases at intervals of 1–3 months as necessary to a typical range of 500–750 mg/day. Because of toxicity, the drug is used rarely today in this setting.
3) Calcium disodium edetate and disodium edetate
The calcium disodium and disodium salt of EDTA (Ethylene Diamine Tetra Acetic Acid) can form stable and water-soluble complex with many metallic ions. The calcium disodium salt has a high affinity for lead, while the disodium salt exhibits a high affinity for calcium.
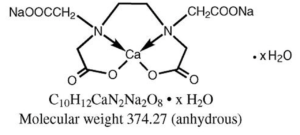
Figure- Calcium Disodium edetate
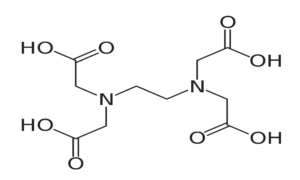
Figure: Disodium edetate
Pharmacokinetics
They are administered through IV or IM route. The drug is distributed in extracellular fluid. They
are excreted completely within 24 hours. Half-life is 20-60 minutes.
Uses
- In treatment of acute and chronic lead poisoning: Disodium calcium edetate exchanges its calcium for lead to form stable. In acute lead poisoning, it is given by slow IV infusion in the dose of 40 mg/kg in two divided doses/day, for a maximum period of 5 days. The course may be repeated after an interval of 2 to 3 days. In children, the dose should not be larger than 55 mg/kg.
- As a diagnostic test for lead poisoning: A total dose of 75 mg per kg divided into 3 doses, is administered as 20% solution by deep IM injection with 1.5% procaine. If the excretion of lead is more than 500 mcg per 24 hours, the ingestion of lead is concluded to be excessive.
- Disodium calcium edetate is used in the treatment of porphyria.
- In poisoning with iron, cadmium and plutonium.
Toxicity
Rapid IV administration can cause hypokalemic tetany. The principal toxic effect is on kidney. Other less serious side effects include malaise, excessive thirst followed by chills and fever, lacrimation etc.
4) Succimer (Dimercaptosuccinic acid or DMSA)
Succimer is chemically similar to dimercaprol and forms stable water-soluble complex with lead, arsenic and mercury. It is administered through oral route. It is indicated for the treatment of lead poisoning, specifically in children with blood lead concentrations higher than 45 micrograms/dL. The most common adverse effects in children and adults were nausea, vomiting, diarrhea, appetite loss, and loose stools. Recommended initial dosage in children is 10 mg/kg every eight hours for five days. The dosage is then reduced to 10 mg/kg every 12 hours for an additional two weeks. DMSA may be considered as an alternative to sodium calcium edetate, particularly when an oral antidote is preferable.
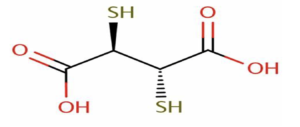
Figure- Structure of succimer
5) Desferrioxamine
It is isolated from Streptomyces pilosus and has high affinity for ferric ion coupled with low affinity for calcium. It binds with ferric ion to form ferrioxamine, a stable and water-soluble complex.
Pharmacokinetics
Desferrioxamine is preferred through parenteral route either IV or IM route. Subcutaneous route is also used It is metabolized by plasma enzymes and excreted in urine.
Uses
- In acute iron intoxication: In severe cases (in shock), the drug is administered by IV infusion: 10-15 mg/kg/hour to a maximum of 80 mg/kg in 24 hours. In less severe cases (without shock), it is given IM in the dose of 40 mg/kg every 4-12 hours.
- Hemochromatosis: It is useful in the prevention and treatment of iron overload in patients with chronic anemia such as in thalassemia major treated with multiple transfusions. In these patients, it is administered by SC infusion 40-50 mg/kg/day over 8-12 hrs for 5-7 days a week. Concomitant oral administration of ascorbic acid (0.5 g twice a day) improves its action. Or 2 g deferoxamine (per unit of blood) should be given by slow intravenous infusion (rate not to exceed 15 mg/kg/h) during the transfusion but not by the same intravenous line through which blood is transfused.
Toxicity
It can cause allergic reaction like rash, pruritus, anaphylaxis. Other adverse effects include abdominal discomfort, diarrhea, tachycardia etc. Chronic administration may cause allergic reactions and cataract formation.
Contraindication
It is contraindicated in patients with severe renal disease or anuria. It is also contraindicated in pregnancy.
References
- Arif Tasleem Jan, Mudasser Azam, Kehkahsan Siddiqui, Arif Ali, Inho Choi, Qazi Mohd. “Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants.” J. Mol. Sci. 16(12): 29592-2963.
- Joel A. Vilensky, Kent Redman. “British anti-Lewisite (dimercaprol): An amazing history.” Annals of Emergency Medicine. 41(3): 378-383.
- Peters RA, Stocken LA, Thompson RH. “British anti-lewisite (BAL).” Nature. 1945; 156: 616-9.
- Scheinberg IH, Sternlieb I. Treatment of the neurologic manifestations of Wilson’s disease. Neurol. 1995 Apr;52(4):339-40.
- Mouret S, Wartelle J, Emorine S, Bertoni M, Nguon N, Cléry-Barraud C, Dorandeu F, Boudry I. Topical efficacy of dimercapto-chelating agents against lewisite-induced skin lesions in SKH-1 hairless mice. Appl. Pharmacol. 2013 Oct 15;272(2):291-8
- M.Walshe. “Penicillamine, a new oral therapy for Wilson’s disease.” The American Journal of Medicine. 21(4): 487-495.
- Park JH, Hill EJ, Chou TH, LeQuire V, Roelofs R, Park CR. “Mechanism of action of penicillamine in the treatment of avian muscular dystrophy.” Annals of the New York Academy of Sciences, 31 Dec 1978. 317:356-369.
- Leckie, W. J. H., Tompsett, S. L. “The Diagnostic and Therapeutic Use of Edathamil Calcium Disodium (EDTA, Versene) in Excessive Inorganic Lead Absorption.” Quarterly Journal of Medicine 27(105): 65-82.
- Mann KV, Travers JD. “ Succimer, an oral lead chelator.” Clinical Pharmacy. 10(12):914-922.
- Sally Bradberry and Allister Vale. “Dimercaptosuccinic acid (succimer; DMSA) in inorganic lead poisoning.” Clinical toxicology. 47(7): 617-631.
- Pharmacology and Pharmacotherapeutics book, R.S. Satoskar. Page no- 1099-1103.
- Goodman and GILLMAN’S Manual of Pharmacology and Therapeutics. Page no- 1126-1140.