- Dantrolene is phenytoin analogue (hydantoin derivative) used in field of neurology and anesthesiology. It is a direct acting postsynaptic skeletal muscle relaxant. Though it is phenytoin analogue, it doesn’t possess antiepileptic properties like phenytoin.
- It was synthesized by Synder and his co-workers in 1967. After its introduction in 1960s, the mortality caused by malignant hyperthermia was decreased from 80% to less than 10%.
Indications of dantrolene
- For treating malignant hyperthermia in children and adults along with general supportive measures. Malignant hyperthermia is severe, life-threatening condition caused by certain volatile anesthetic agent or neuromuscular blocking agent like succinylcholine and is characterized by heat production from skeletal muscle which causes hyperthermia, rigidity and contracture of skeletal muscle, tachycardia, and metabolic acidosis. If not treated in time, it can cause circulatory collapse and death.
- Used before or after operation or surgery to prevent development of clinical signs of malignant hyperthermia in individuals susceptible to malignant hyperthermia. Such individuals include burn victims, patients with myopathy, myotonia, muscular dystrophy and osteogenesis.
- Used in muscle spasticity disorder (especially cerebral spasticity).
- Some of its off label uses include:
- Treatment of neuroleptic malignant syndrome.
- In overdose of 2,4-dinitrophenol.
- Its use for Alzheimer’s disease and vasospasm due to aneurysmal subarachnoid hemorrhage is being researched.
Mechanism of action of dantrolene
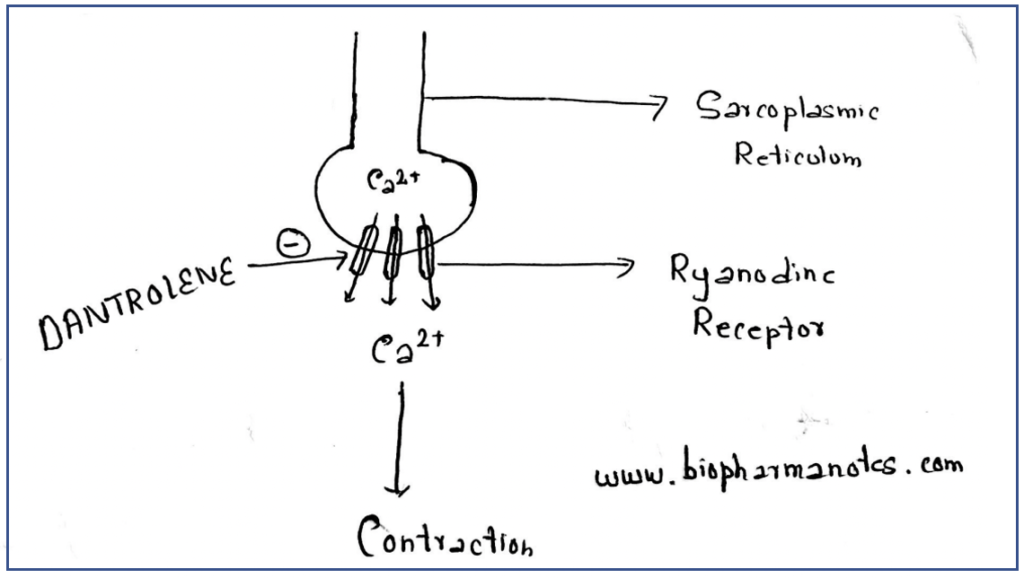
Figure- Mechanism of action of dantrolene
- The exact mechanism of action and molecular targets of dantrolene is not known in detail.
- Various studies suggest that ryanodine receptors are binding site of dantrolene. It binds to and antagonizes ryanodine receptors which are important calcium release channel present in skeletal muscle sarcoplasmic reticulum. The release of calcium from ryanodine receptors causes muscle contraction.
- Binding of dantrolene to ryanodine receptors prevent the release of calcium from sarcoplasmic stores which prevent the binding of calcium to troponin on actin filaments and inhibit action and myosin cross-bridging. This results in prevention of muscle contraction and decrease of energy expense in muscle cells.
Pharmacokinetics of dantrolene
- It is administered via oral and IV route. The route of administration is decided by its intended use. IV route is preferred if used for malignant hyperthermia and oral route is preferred if used for spasticity disorder. For IV use, it is available as lyophilized dantrolene sodium, 20 mg in vials added to 3 gm mannitol to improve water solubility. When given through oral route, it is incompletely absorbed.
- It mainly affects skeletal muscle and has very less effect on cardiac and smooth muscle. Its bioavailability is around 70%.
- It is metabolized in liver. The major metabolite is 5- hydroxy dantrolene and acetylamino metabolite of dantrolene.
Adverse effects
- Common side effects include dizziness, drowsiness, fatigue, diarrhea, and muscle weakness which occur in around 75% of patients. It can increase serum potassium level.
- It can cause hepatotoxicity rarely. Oral dantrolene has black box warning for hepatotoxicity. So, patients on oral dantrolene therapy should be continuously monitored before starting and during the dantrolene therapy.
- Some other rare and very serious side effects include respiratory depression, pleuropericardial reaction, lymphocytic lymphoma, and seizures.
Drug Interactions
- Concurrent administration with calcium channel blockers like verapamil and nifedipine may increase risk or severity of hyperkalemia and deVere cardiovascular collapse.
- It may potentiate neuromuscular blockade caused by vecuronium.
Contraindications
- There are no known contraindications for IV dantrolene except hypersensitivity to dantrolene.
- For oral route, the contraindications include:
- Patients with hepatic impairment including non-alcoholic steatohepatitis, cirrhosis, hepatitis B or C infections.
- Pediatric patients of age > 5 years.
- Patient with known hypersensitivity to dantrolene.
- Patient with cardiovascular impairment.
- It is pregnancy category C drug (no adequate studies in human). Hence, it is used in pregnancy only if clearly needed.
- It is contraindicated in breastfeeding female. If it is needed to be administered in breastfeeding women, breastfeeding should be terminated.
References
- https://www.ncbi.nlm.nih.gov/books/NBK535398/
- https://www.ncbi.nlm.nih.gov/books/NBK535398/
- https://go.drugbank.com/articles/A2144
- https://chemm.nlm.nih.gov/pregnancycategories.htm
- https://en.wikipedia.org/wiki/Dantrolene
- https://www.rxlist.com/dantrium-drug.htm#interactions
- Meyler’s Side Effects of Drugs (Sixteenth Edition). The International Encyclopedia of Adverse Drug Reactions and Interactions. 2016, Pages 822-823.
- Krause T et al. Dantrolene – A review of its pharmacology, therapeutic use and new developments. Anaestheisa. 2004; 59(40; 364- 373.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.