- Lamotrigine is 2nd generation antiepileptic drug which belongs to phenyltriazine class.
- It is included in World Health Organization’s List of Essential Medicines. It was developed by Glaxosmithkline and has been on market since 1994.
Indications of lamotrigine
- It is a broad-spectrum antiepileptic drug. Used for treating partial seizures, primary generalized tonic- clonic seizures and generalized seizures due to Lennox- Gastaut syndrome and absence seizure. It is effective as add on therapy as well as monotherapy.
- Used for maintenance treatment of bipolar I disorder to prevent recurrence of mood episodes (which includes mania, hypomania, depression and mixed episodes).
- Off- label uses include panic disorder, binge eating disorder and basilar migraine.
Mechanism of action of Lamotrigine
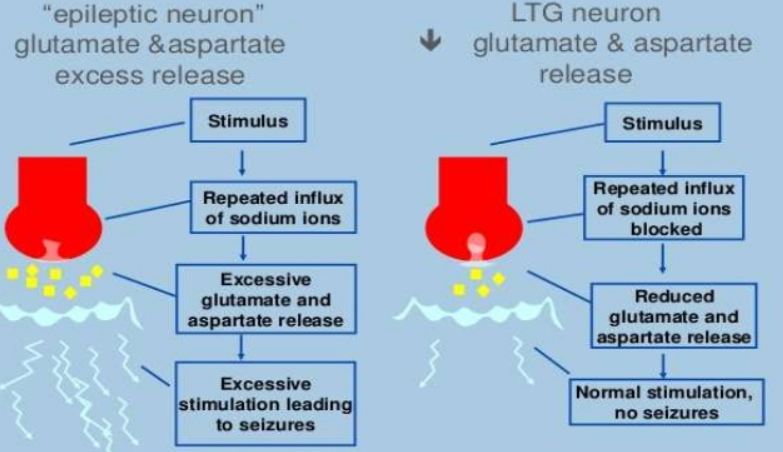
Figure- Mechanism of action of Lamotrigine (Source- slideshare.com)
- Its mechanism of action is similar to that of phenytoin and carbamazepine.
- It binds to inactive sodium channels, suppress the release of excitatory amino acid glutamate and inhibit sodium current. Reduction in neuronal Na+ concentration decreases post-tetanic potentiation (PTP) which is responsible for spread of seizure activity and inhibit spread of seizure discharge in the brain and shortens the duration of after discharge.
- It also enhances action of gamma amino-butyric acid, an inhibitory neurotransmitter. It results in reduction of pain-related transmission of signals along nerve fibers.
Pharmacokinetics of Lamotrigine
- It is well absorbed after oral administration. Available as tablets, chewable tablets and orally disintegrating tablets. The bioavailability of oral formulation is about 98%.
- Metabolism takes place by UGT1A4 pathway to 2-N-glucuronide metabolite it undergoes complete metabolism in liver. Its half-life is around 24 hours. It is excreted in both urine and feces. When administered in hepatic and renal patients, close monitoring is required.
Adverse effects
- Common side effects include skin rashes, nausea, vomiting, diplopia and ataxia. It can cause sleepiness, dizziness, constipation, abdominal pain and edema.
- Few cases of Steven- Johnson syndrome and disseminated intravascular coagulation is also reported. Skin rashes may be severe in children and may require withdrawal.
Drug Interactions
- When used with valproate, it inhibits glucuronidation of lamotrigine and increases its plasma level. Lamotrigine lowers valproate level. So, when used in combination with valproate, the dose of lamotrigine should be reduced to half. When lamotrigine is added to regimen of valproate therapy, it should be titrated slowly to prevent risk of serious rash.
- Its half-life is reduced by drugs like phenytoin, carbamazepine, rifampin, ritonavir, primidone and phenobarbitone.
Contraindications/ Warning
- Contraindicated in patients hypersensitive to lamotrigine.
- While discontinuing lamotrigine, it should not be stopped suddenly as it may cause withdrawal seizures. It should be stopped in stepwise way over the period of two weeks.
- It is pregnancy category C drug. Animal studies have shown risk of fetal malformations.
References
- https://go.drugbank.com/drugs/DB00555
- https://www.statpearls.com/articlelibrary/viewarticle/24015/
- Rambeck B, Wolf P. Lamotrigine Clinical Pharmacokinetics. Clinical Pharmacokinetics. 1993; 25: 433-443.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Essentials of Medical Pharmacology. 7th edition.