What is Alzheimer?
Alzheimer’s disease is a progressive neurodegenerative disorder resulting in progressive cognitive impairment and loss of short-term memory. It is mostly seen in old age people. Some of the most common symptoms include loss of short-term memory, impairment in ability to calculate, to remember directions and to perform day-to day activities like using common objects and tools. Ultimately this all leads to major functional and behavioral disabilities. AD patients frequently exhibit non-cognitive symptoms like depression, anxiety and aggressiveness which worsen as the disease progresses. Death, which may occur due to complication of immobility such as pneumonia or pulmonary embolism, usually occur within 6-12 years of onset.
Millions of people worldwide are affected by Alzheimer’s disease with approximately 500000 in USA. It is the sixth leading cause of death in USA. In 2012, costs associated with care of AD patients were estimated at US$200 billion, not including contributions from unpaid caregivers valued at over US$210 billion. The global prevalence is projected to increase to 115 million patients by 2050.

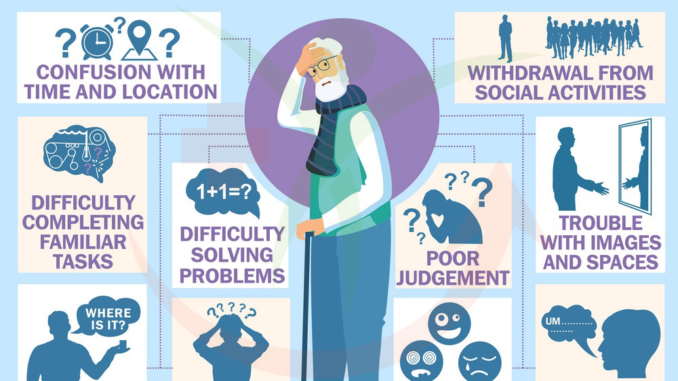
Figure 1: Symptoms of Alzheimer
Pathophysiology of Alzheimer
According to post-mortem examination of AD brains and imaging procedures in AD patients, considerable atrophy particularly of the forebrain and hippocampus is observed with deficiency of important neurotransmitter pathways. The pathological hallmarks of AD are extracellular senile plaques which are formed from accumulation of β- amyloid protein and intracellular neurofibrillary tangles. According to current theories, amyloid protein forms into toxic amyloid oligomers and fibrillar aggregates that promote the development of tau hyperphosphorylation, ultimately resulting in neuronal dysfunction and death.
Intracellular neurofibrillary tangles along with Aβ favor the excessive accumulation of reactive oxygen species (ROS) due to mitochondrial dysfunction which increases oxidative stress leading to increased levels of lipid peroxidation, protein oxidation and DNA/RNA oxidation. This ultimately results in neurodegeneration and cerebral amyloid angiopathy (CAA). ROS can also increase the production and aggregation of Aβ and promote the phosphorylation of tau, thus establishing a vicious cycle of pathogenesis in AD.
Degeneration of cholinergic neurons have been implicated in pathogenesis of AD. The brains of patients with Alzheimer’s disease show a reduction in acetylcholinesterase, the enzyme in the brain that is primarily responsible for the hydrolysis of acetylcholine. This is mainly due to the depletion of cholinesterase-positive neurons within the cerebral cortex and basal forebrain.

Figure 2: Pathophysiology of Alzheimer
Role of exercise in prevention and treatment of Alzheimer’s Disease
There is no cure for Alzheimer’s Disease. Medications are available which temporarily reduce some symptoms or slows progression of disease in some people. Inhibitors of acetylcholinesterase like donepezil, rivastigmine, tacrine and galantamine are approved by FDA for treatment of Alzheimer’s disease. Memantine which is NMDA receptor antagonist is also licensed for treatment of AD. Drug therapy should be combined with non-pharmacological treatment.
Regular exercises like jogging, swimming, stretching may prevent the progression of brain disorder and other metabolic diseases like diabetes, hypertension and obesity which are risk factors for Alzheimer’s disease. There have been various studies which proves that physical exercises are beneficial in both prevention and treatment of AD. Regular physical exercises increase energy metabolism, neurotrophin synthesis and enhance endurance of cell and tissues to oxidative stress. Regular exercise is also beneficial in learning and memory loss caused by sleep deprivation, maternal deprivation stress and addiction.
According to one study, 40 min of physical activities such as the treadmill and stair-climbing activities over a period of 12 consecutive weeks, is found to be associated with an increase of cerebral blood flow that improves neurogenesis in brain areas, including dentate gyrus of the hippocampus. The increase in cerebral blood flow is associated with the reduction of cerebrovascular and endothelial dysfunction resulting in the building of vascular reserve and the maintenance of neuronal plasticity.
Physical activity also helps to counteract the noxious effects of oxidative stress in animal models which is related with the reduction of cholesterol and insulin resistance. This results in increased vascularization and improved energy metabolism such as glucose metabolism and also facilitates neurogenesis and synaptogenesis, which leads in improvement of memory and cognitive functions
Doing regular exercise prevents the increase in level of AD related proteins in hippocampus areas of brain. BDNF (Brain Derived Neurotropic Factor) is the prominent mediator of effect of exercise on hippocampus. BDNF is a member of the nerve growth factor family and play important role in neuronal development and synaptic plasticity. BDNF reduces toxic Aβ plaques by upregulating α-secretase activity. Exogenous administration of BDNF cannot be used as option for treatment of AD because of its low blood brain barrier penetration and poor biostability.
Regular exercise has beneficial effect on both cognitive and non-cognitive functions in humans and experimental animals. Non-cognitive functions are reduced due to reduction in AD-induced oxidative stress burden.
Therefore, exercise could be a reasonably safe and inexpensive approach for the management of AD through its unique ability to exquisitely increase BDNF levels in the brain, reduce AD-induced oxidative stress, improve vascularization and improve synaptic plasticity.
References
- Role of physical exercise in Alzheimer’s disease. Wei-Wei Chen, Xia Zhang, Wen- Zuan Huang. Biomed Rep. 4(4):403-407.
- Delayed effects of combined stress and Aβ infusion on L-LTP of the dentate gyrus: Prevention by nicotine. Karim A. Alkadhi. Neuroscience Letters. 682:10-15.
- Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Karim A. Alkadhi, An T. Dao. Molecular and Cellular Neuroscience. 86 (2018): 25–29.
- Chronic Treatment with DCPCX, an Adenosine A1 Antagonist, Worsens Long-Term Memory. Craig Vollert, Gloria S. Forkuo, Richard A. Bond, and Jason L. Eriksen. Neurosci Lett. 2013 August 26; 548: 296–300.
- A novel function for proSAAS as an amyloid anti-aggregant in Alzheimer’s disease. Akina Hoshino, Michael Helwig, Sina Rezaei, Casey Berridge, Jason L. Eriksen2, and Iris Lindberg. J Neurochem. 2014 February; 128(3): 419–430.
- Regular exercise prevents non-cognitive disturbances in a rat model of Alzheimer’s disease. An T. Dao, Munder A. Zagaar, Samina Salim, Jason L. Eriksen, and Karim A. Alkadhi. Int J Neuropsychopharmacol. 2014 April; 17(4): 593–602.
- Inhibitory Neuron and Hippocampal Circuit Dysfunction in an Aged Mouse Model of Alzheimer’s Disease. Anupam Hazra, Feng Gu, Ahmad Aulakh, Casey Berridge, Jason L. Eriksen, Joku¯ bas Zˇ iburkus. PLOS ONE. 8(5): 1-9.
- Pharmacology and Pharmacotherapeutics book, R.S. Satoskar. Page no- 254-255.
- Goodman and GILLMAN’S Manual of Pharmacology and Therapeutics. Page no- 344-345.
- A Textbook of Clinical Pharmacology and Therapeutics. Page No- 131-132.