Why are animal toxicity studies required?
Different types of toxicity studies should be carried out for evaluation of toxic effects of therapeutic agents or potential toxicants that may be harmful to human and animal life. Toxicity studies of therapeutic agents are carried out before clinical trials and may involve animals like mice, rats, guinea pig etc. Current testing regimes have evolved significantly over the past three decades. Toxicity has two main components: the effect caused and the level of exposure (dose) at which the effect is observed. Some tests are designed specifically to detect particular effect like skin and eye irritancy, skin sensitization and mutagenicity studies. Other tests (such as sub-chronic and chronic studies) are used to detect wide range of less specific effect on different organs or systems of body.
Information from toxicity tests is used to provide a classification for a chemical, for example to assign appropriate warning labels for containers, and, where necessary, for selecting measures, such as protective equipment, during manufacture, exposure and use.
The important tests which need to be performed are:
- Systemic toxicity studies, a) With a single dose and b) With repeated doses
- Specialized toxicity studies
- Local toxicity studies
Systemic toxicity studies
a) Single dose toxicity studies: In this study, a drug is tested for the effects of a single dose. Historically, a primary objective of acute toxicity testing was to determine an LD50 dose, or that dose which would be lethal to 50% of the animals treated. It is also used to determine minimum lethal dose, maximum tolerated dose and targeted organ of toxicity. It is assessed by administering single dose of compound to rats or mice often numbering 10 or more per sex. Route of administration is same as that intended for human. Animals are observed for mortality for up to 14 days and for 72 days if the compound is administered through parenteral route. The effect of drugs on important physiological functions of body and body weight is also studied. Depending on the rate of survival in the initial group(s), additional groups are added to the study at higher and/or lower doses such that most animals that receive the highest doses die and most that receive the lowest doses survive.
The choice of routes of administration depends on the intended clinical route and on how much is already known about the oral bioavailability of the test substance. At least one parenteral route is used for acute testing, and that route may be intravenous (IV) if the product is soluble in vehicle (e.g., water or saline) or IP as a suspension if the product is insoluble in an aqueous vehicle. If the intended clinical route is not oral, the oral route is usually selected as a secondary route for acute toxicity testing to provide information regarding accidental oral ingestion.
b) Repeated dose toxicity studies:
These has three main objectives: to identify toxicity that occurs only due to continuous exposure to compound, to determine doses at which such effects occur and to find out organs most affected. Repeated-dose studies are conducted for various periods of time. Repeat-dose studies can involve durations of 14 days, 28 days as well as 1, 3, and 6–9 months. It should be conducted in at least two species and one of them should be non-rodent. Dogs are the usual nonrodent species in most cases. In some cases, rabbits and minipigs are also used.
Three doses are selected: (1) the highest dose having observable toxicity; (2) mid-dose causing some symptoms but no gross toxicity or death; and (3) the lowest dose free of toxicity and comparable to the intended therapeutic dose. A control group should be treated with vehicle for comparison. The route of drug administration and duration between doses should be the same as that proposed for human use.
Parameters which should be observed are eye changes, loss of fur, behavioral and physiological observations, body weight changes, food/water intake, blood chemistry, hematology and examination of organs. The sites of injections are inspected for gross and microscopic changes. ECG and fundoscopy are done in non-rodent species. Finally, highest dose without significant effects (the ‘no observed adverse effect level’, or NOAEL) is accessed. Sometimes, ‘high-dose reversal study’ is included wherein animals are studied after stopping the treatment or after recovery from signs of toxicity.
Specialized Toxicity Studies
a) Female reproduction and fetal developmental toxicity studies
The effects of compounds on various aspects of the reproductive capacity of the adult like mating behavior, gestation development, and on the development of the offspring, health during pregnancy are determined. It is carried out on one rodent and one non-rodent species. Repeated oral dose of compound is administered to young rats through the period of sexual maturation into young adulthood when the animals are mated to treated females. The females are dosed throughout pregnancy and until the offspring are weaned. The pups are dosed until adulthood and mated, and the second-generation young evaluated.
Teratogenic effect of drug (ability of the drug to induce fetal malformations and/or death in utero) is determined if present. If the drug is intended to be used in pregnant or nursing mothers, perinatal toxicity study is performed. Typically, groups of pregnant rats or rabbits are treated orally for up to the whole period of gestation, and the uterine contents are then examined and evaluated just prior to parturition.
b) Genotoxicity studies
The interaction of drugs with genetic materials like DNA or chromosomes should also be studied.
In beginning of this study, in-vitro assays are performed for mutation in bacteria or isolated mammalian cells. If one or more of these in-vitro tests results came positive, animal studies usually on mouse are conducted. In practice, very few chemicals that have been confirmed to be mutagenic in-vitro are tested any further in animals. However, in the case of pharmaceuticals, regulatory requirements demand that an in-vivo test be completed before the start of Phase II clinical studies in humans.
In vivo tests include the rodent bone marrow micronucleus test in which a single dose of compound is administered to rats or mice which are killed either 24 or 48 hours later for examination of chromosomal changes in bone marrow cells. Different assays used are comet assay, micronucleus assay, chromosome aberration assay, gamma-H2AX, and bacterial reverse testing.
c) Carcinogenicity studies
The objectives of carcinogenicity studies are to identify a tumorigenic potential in animals and to assess the relevant risk in human. The drug which are intended to be used frequently or to be used for more than 6 months are mostly tested for this. Pharmaceuticals administered infrequently or for short duration of exposure (e.g. , anesthetics and radiolabeled imaging agents) do not need carcinogenicity studies unless there is cause for concern. Ideally, the route of administration selected for carcinogenicity studies should be the intended clinical route.
Carcinogenicity studies should be recommended for drugs if there is concern about their carcinogenic potential. Factors to consider may include:
– Previous demonstration of carcinogenic potential in the product class that is considered relevant to humans.
– Structure–activity relationship suggesting carcinogenic risk.
– Evidence of preneoplastic lesions in repeated-dose toxicity studies.
– Long-term tissue retention of parent compound or metabolite(s) resulting in local tissue reactions or other pathophysiological responses.
– Genotoxicity: Unequivocally genotoxic compounds, in the absence of other data, are presumed to be trans-species carcinogens, implying a hazard to humans.
d) Allergenicity/ Hypersensitivity Studies
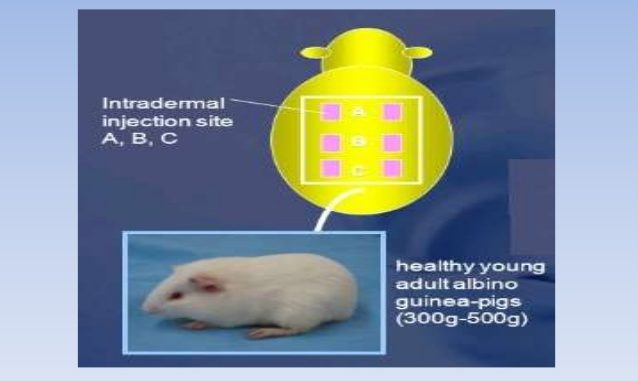
These tests are carried out either in guinea pigs or mice. Guinea pig maximization test is used to determine minimum irritant dose or maximum non-irritant dose. Component called the Complete Freund’s Adjuvant (FCA) is used. FCA has tendency to release antigens steadily. The experimental material is intradermally injected with FCA and evaluated after 7 days. On the 7th day, same substance is topically applied for the next 2 days. This enhances the sensitivity of the test. After 14 days, skin reactions are evaluated.

Figure- Guinea pig maximization test
Local lymph node assay is performed on mice (either male or female). Drug treatment is given on ear skin and auricular lymph node is dissected after 5 days and increase in 3H- thymidine is used for evaluation.

Figure- Local lymph node assay
e) Male Fertility Studies
In this, the effect of drug on structure and functions of male reproductive organs are studied.
Local Toxicity Studies
The drug is applied to appropriate side like eye, ear, vagina, rectum to determine local effect in suitable species (mice or guinea pig). In vaginal toxicity studies swelling in vagina and histopathology of vaginal wall should be observed. In rectal toxicity studies, signs of pain, blood or mucous and histopathology of rectal mucosa should be examined. In ocular toxicity studies, changes in cornea, iris and aqueous humor and histopathology of eye should be examined. For parenteral drugs, site of injection should be examined grossly and microscopically. Respiration rate and histological examination of respiratory passages and lung tissues should be examined in inhalation toxicity studies.
References
- Seung Hun Kang, Jee Young Kwon, Jong Kwon Lee, and Young Rok Seo. Recent Advances in In Vivo Genotoxicity Testing: Prediction of Carcinogenic Potential Using Comet and Micronucleus Assay in Animal Models. J Cancer Prev. 2013 Dec; 18(4): 277–288.
- Saganuwan S A. Toxicity studies of drugs and chemicals in animals: An overview. J. Vet. Med. 20 (4): 291-318.
- Biomarkers in Toxicology. Page no- 7-73.
- A comprehensive guide to toxicology in non-clinical drug development, 2nd
- A comprehensive guide to toxicology in pre-clinical drug development, page no- 713-723.
- The Nonhuman Primate in Nonclinical Drug Development and Safety Assessment.
- The ethics of research involving animals. Page no- 155-167.
- Pharmacology and Pharmacotherapeutics. 81-83.