- Tacrolimus is macrolide antibiotic and is commonly used as immunosuppressive agent. It was discovered in 1984 in fermentation broth of Japanese sample soil containing bacteria Streptomyces tsukubaensis. It was approved by FDA for its use in liver transplantation in 1994.
- Tacrolimus is most frequently used when compared to other immunosuppressant due to better safety profile and increased long-term survival. It is around 100 times more potent than cyclosporine.
Indications of tacrolimus
- Used to prevent organ transplant rejection due to its immunosuppressive properties.
- Used to treat moderate to severe atopic dermatitis.
- In treating severe refractory uveitis after bone marrow transplant.
- Used to treat skin condition vitiligo.
- Its off-label uses include crohn disease, myasthenia gravis, rheumatoid arthritis and graft-versus-hos disease (GVHD).
Mechanism of action of tacrolimus
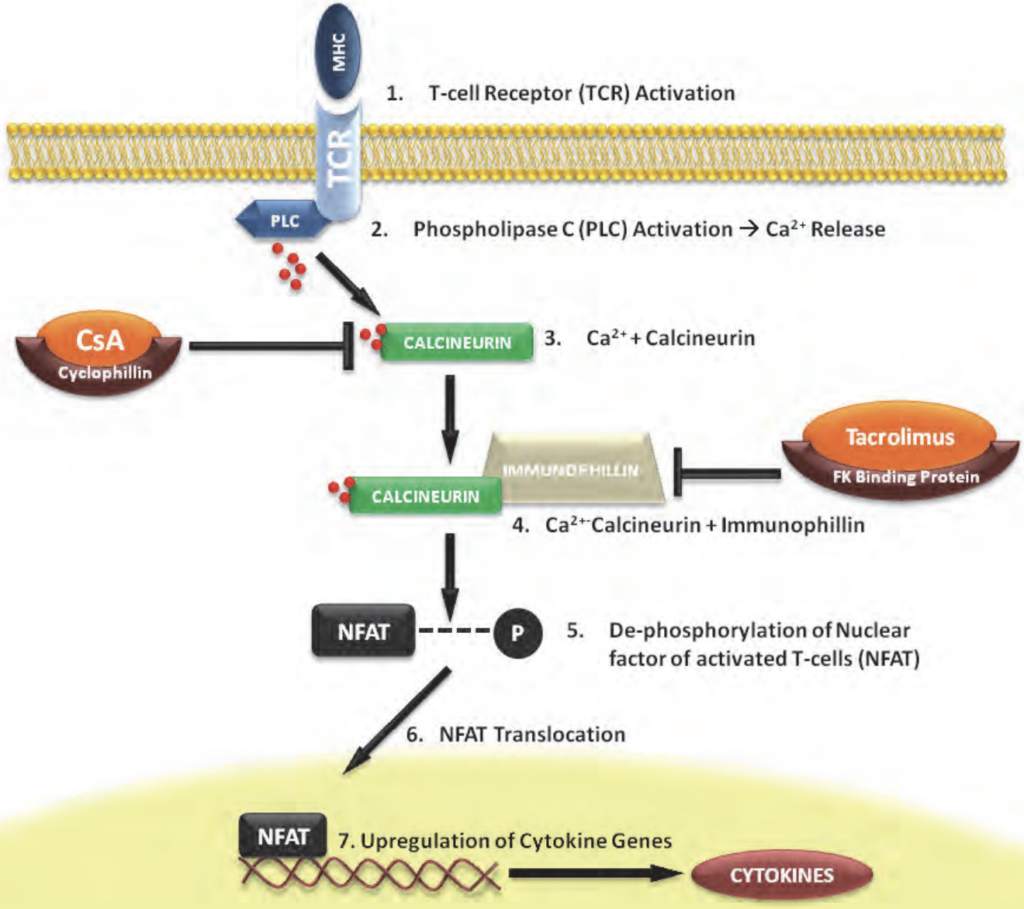
Figure- Mechanism of action of Tacrolimus (Source- Slattery et al, 2012)
- It is calcineurin inhibitor. It binds to FK506 binding protein- 12 (FKBP-12). FKBP-12 is an immunophilin structurally related to cyclophilin. The tacrolimus- FKBP then forms complex with calcium, calmodulin and calcineurin which inhibit phosphatase activity of calcineurin.
- The inhibition of phosphatase activity of calcineurin prevents dephosphorylation and nuclear translocation of nuclear factor of activated T- cells (NFAT) leading to inhibition of T cell activation. This inhibition of T cell activation results in immunosuppressive action of tacrolimus.
Pharmacokinetics of tacrolimus
- It is available for oral administration and is also available as sterile solution for injection. Food especially high fat or high carbohydrate meals may decrease absorption of tacrolimus. Its GI absorption is incomplete and variable. Its oral form is available as both immediate release and extended-release formulation.
- It binds extensively with plasma proteins (around 75-99%) like albumin and a-1 acid glycoprotein. It is extensively metabolized in liver by CYP3A4/5 isoenzymes. Half-life is around 12 hours.
- The parent drug and its metabolites excreted via feces. Less than 1% is excreted unchanged.
Adverse effects
- It can cause severe nephrotoxicity. It can also cause neurotoxic effects, nausea, diarrhea, hypertension, hyperkalemia, hyperglycemia, and hypomagnesemia. Tacrolimus has higher chances of causing diabetes compared to cyclosporine.
- It can cause dermatologic effects like alopecia, rash, pruritus, and acne vulgaris. Other adverse effects include blurred vision, visual disturbance, tinnitus, muscle cramps, arthralgia etc.
- As it causes immunosuppression, it increases chances of other opportunistic infections and secondary tumors.
Drug Interaction
- Co-administration with cyclosporine (another immunosuppressant agent) may cause additive or synergistic nephrotoxicity. Hence, while switching from cyclosporine to tacrolimus or vice versa, at least 24 hours gap is required.
- As it is metabolized by CYP3A system, it may interact with drugs which are substrate of CYP3A. Drugs which inhibit CYP3A enzymes.
Contraindication
Contraindicated in following conditions:
- In patients hypersensitive to tacrolimus.
- Immunosuppressive patients.
- Contraindicated in pregnancy and breast-feeding women.
- Contraindicated in patients with QT interval prolongation.
- It is avoided in patients with neoplastic disease.
References
- https://www.statpearls.com/ArticleLibrary/viewarticle/29856
- https://go.drugbank.com/drugs/DB00864
- https://en.wikipedia.org/wiki/Tacrolimus
- Dheer D. Tacrolimus: An updated review on delivering strategies for multifarious diseases. European Journal of Pharmaceutical Sciences. 2017; 114: 217-227
- Plosker GL. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000 Feb; 59(2): 323-89.
- Pharmacology and Therapeutics for Dentistry (Seventh Edition). 2017; 504-529.
- Slattery C et al. Mechanisms of Calcineurin Inhibitor Nephrotoxicity in Chronic Allograft Injury. Chronic Kidney Disease and Renal Transplantation, 2012.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Essentials of Medical Pharmacology. 7th edition.