Remdesivir is an investigational anti-viral compound undergoing clinical trials in China, the United states and the United Kingdom as a potential treatment for COVID-19. Today, FDA approved Remdesivir for emergency use to treat COVID-19.
- Generic Name- Remdesivir
- Producer Company- Gilead Sciences, Inc.
It was originally developed to treat Ebola in response to the 2014 West African Ebola virus epidemic. Remdesivir has demonstrated in vitro and in vivo activity in animal models against the viral pathogens that cause MERS and SARS, which are coronaviruses structurally like SARS-CoV-2, the coronavirus that causes COVID-19.
Possible Mechanism of action of Remdesivir
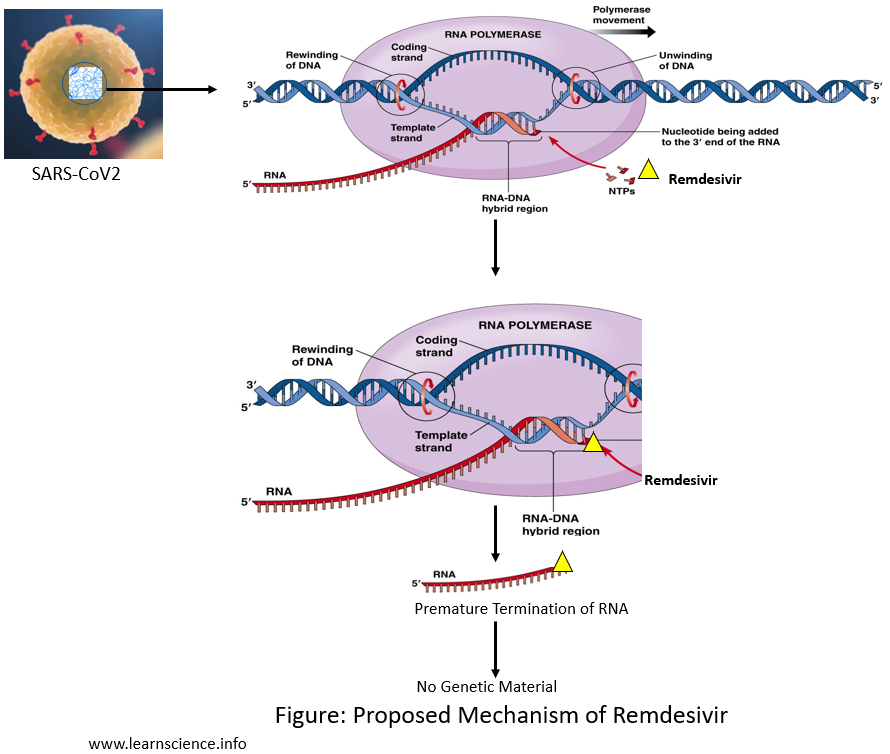
- Remdesivir is an adenosine analogue, which incorporate into nascent viral RNA strand and results in pre-mature termination.
- Corona virus replicate by copying their genetic material (RNA). In this process, an enzyme called RNA- dependent RNA polymerase incorporate the incoming nucleotide in the growing strand of RNA.
- Remdesivir triphosphate (RDV-TP) compete with its natural counterpart ATP and gets incorporated into RNA. Once added into the growing RNA strand, the inhibitor does not cause immediate chain termination. The presence of the 3′-hydroxyl group allows the addition of three more nucleotides until RNA synthesis is arrested. Thus, delayed chain termination may be a plausible mechanism of action.
References
- https://www.fda.gov/media/137564/download
- https://www.gilead.com/purpose/advancing-global-health/covid-19/remdesivir-clinical-trials
- Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Tchesnokov EP1,2, Feng JY3, Porter DP4, Götte M56.