- Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are anti-viral agents used to treat HIV (Human Immunodeficiency Virus).
- HIV infection and AIDS (Acquired Immunodeficiency Syndrome) is caused by retrovirus, Human Immunodeficiency Virus (HIV 1 and 2) and is most transmitted by sexual intercourse. It is also transmitted by use of contaminated syringe or blades and by blood transfusion.
- The first NNRTI- Nevirapine was approved by FDA in 1996. Other approved NNRTIs:
- Efavirenz
- Delavirdine
- Etravirine
- Rilpivirine.
- Nevirapine, Efavirenz and Delavirdine are first generation NNRTIs and have low genetic barrier and poor resistance profile. Etravirine and rilpivirine are 2nd generation NNRTIs.
Mechanism of action of Non-Nucleoside Reverse Transcriptase Inhibitors
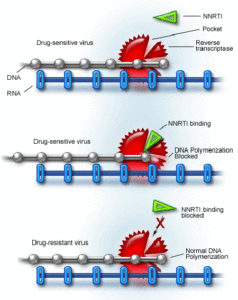
Figure 1- Mechanism of action of NNRTIs
- They are highly selective, non-competitive inhibitors of HIV-1 reverse transcriptase (RT). Reverse transcriptase is an important enzyme that control replication of the genetic materials of the HIV. It is used by HIV virus to convert its RNA to DNA (reverse transcription).
- NNRTIs bind to allosteric hydrophobic site of RT, adjacent to active site and induce conformational change in enzyme resulting in enzyme inhibition. This results in reduction of viral DNA production. As their binding site is strain specific, they are only active against HIV-1 but not against HIV-2 and other retroviruses.
- They don’t require activation by cellular enzymes. They don’t affect host cell DNA polymerases.
Resistance
- Chances of developing resistance is rapid (within few days or weeks). Single codon mutation can reduce susceptibility to these drugs by 100 times.
- There are chances of cross-resistance with other NNRTIs. Therefore, any patient who develop resistance to any one NNRTI may also develop resistance to other NNRTIs and is considered as have failed the whole class of NNRTIs.
Therapeutic Uses of Non-Nucleoside Reverse Transcriptase Inhibitors
- They are used in combination therapy with other anti-retroviral agents like NRTIs (Nucleoside Reverse Transcriptase Inhibitors), PI (Protease Inhibitors) to treat HIV and AIDS.
- Nevirapine is used to treat mother to child transmission of HIV-1.
Pharmacokinetics of Non-Nucleoside Reverse Transcriptase Inhibitors
- Each agent has different pharmacokinetic properties.
- They are mostly administered orally and rapidly absorbed. And their absorption is not affected by food. They are metabolized in liver.
| Parameters | Nevirapine | Efavirenz | Delavirdine |
| Oral Bioavailability | 90-93 | 50 | 85 |
| Effect of meals on AUC | No effect | ↑17–28% | No effect |
| Excretion | In urine | In urine and feces | In urine |
| Plasma t1/2, h | 25-30 | 40-55 | 2-11 |
| Plasma protein binding, % | 60 | 99 | 98 |
| Autoinduction of metabolism | Yes | Yes | No |
| Inhibition of CYP3A | No | Yes | Yes |
Table 1- Pharmacokinetic properties of some NNRTIs
- The bioavailability of etravirine is enhanced by high-fat diet. Its half-life is around 40 hours and is indicated for twice daily dosing. Excretion takes place mainly via feces. It is potent inducer of CYP 450 enzymes.
- Rilpivirine is administered once per day with meal. It is highly bound to plasma proteins mainly albumin. It is excreted mainly in feces.
- Efavirenz crosses the BBB, which another anti-retroviral drug cannot do.
Adverse Effects
- The most common adverse effect are hypersensitivity reactions including rash occurring in about 15-25% of patients. Severe dermatologic effects include Steven-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN). Other side effects include myalgia, arthralgia and lipodystrophy.
- Efavirenz is associated with CNS side effects like headache, dizziness, vivid dreams and loss of concentration. They are resolved within a few weeks.
- Common adverse effects of rilpivirine are depressive disorders, insomnia and headache.
- Nevirapine is associated with severe and fatal hepatitis and increase in hepatic transaminases level. This is more common in women, specially during pregnancy.
Drug Interactions
- Nevirapine is an inducer of CYP3A4 isoenzymes and increase metabolism of drugs including oral contraceptives, ketoconazole, methadone, quinidine and warfarin.
- Etravirine and efavirenz are potent inducer of CYP 450 enzymes and reduce concentration of drugs that are substrate of CYP 450 enzymes.
- Concurrent use of rifabutin and fosamprenavir decrease the level of delavirdine.
Contraindications
- Efavirenz, delavirdine are teratogenic and are avoided in pregnant women.
References
- Smith PF, DiCenzo R, Morse GD. Clinical Pharmacokinetics of Non-Nucleoside Reverse Transcriptase Inhibitors. Clinical Pharmacokinetics. 2001; 40: 893–905.
- Sluis-Cremer N, Tachedjian G. Mechanisms of inhibition of HIV replication by nonnucleoside reverse transcriptase inhibitors. Virus Res. 2008; 134(1-2): 147–156.
- Usach I, Melis V, Peris JE. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. JIAS. 2013; 16: 18567.
- Pharmacology and Pharmacotherapeutics book. 24th edition.
- Goodman and Gillman’s Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology. 6th edition.
- A Textbook of Clinical Pharmacology and Therapeutics. 5th edition.