What is Griseofulvin?
- Griseofulvin is a fungistatic agent used to treat various fungal infection. It is isolated from Penicillium griseofulvium.
- It was first discovered in 1939. Griseofulvin is available both as generic and brand medicine. It is included in World Health Organization’s List of Essential Medicines.
Anti-fungal activity of Griseofulvin
- Griseofulvin acts as fungistatic invitro for various dermatophytes. It inhibits growth of various species of Trichophyton, Epidermophyton and Microsporum.
- It doesn’t act against any bacteria, C. albicans or deep fungi.
Why it is used?
It is used to treat fungal infections. However, its use has decreased due to availability of more potent anti-fungal agents. Due to its narrow spectrum and high cost, it is recommended that it should not be used unless cheaper and safe topical therapy fails to treat the infection.
Its use are as follows:
- To treat Tinea capitis– ringworm of scalp.
- Tinea cruris and Tinea corporis– ringworm of the groin and body.
- To treat Tinea pedis and Tinea manus– ringworm of the feet (athletes’ foot) and hands.
- Tinea barbae– fungal infection of beard caused by T. faviforme, T. mentagrophytes and M. canis.
- To treat onychomycosis (fungal infection in nails of hands and toe).
How it works?
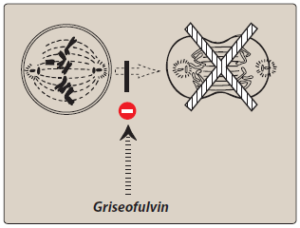
Figure- Inhibition of mitosis by griseofulvin (Source- Lippincott’s Illustrated Reviews)
- It binds with polymerized microtubule and disrupt the mitotic spindle resulting in inhibition of fungal mitosis. It may also inhibit fungal DNA replication.
- Dermatophytes mainly attack keratinous tissue. Griseofulvin binds with keratinocyte precursor cells and makes new keratin resistant to fungal invasion. The new growth of hairs and nails is the first to be free from fungal infection. It doesn’t affect already established fungus so, it takes long time to achieve complete cure.
Pharmacokinetics of Griseofulvin
- Micronized and ultramicronized powders are used to facilitate dissolution as small particles are better absorbed than large particles. Divided oral dose achieve higher level than a single dose. Presence of high- fat food promote its absorption.
- It reaches the skin through sweat and its hydrophobic properties allow it to concentrate in hair follicle and stratum corneum. It is detectable in the stratum corneum within 4-8 hours of oral administration. Small amount is present in body tissues and fluids.
- Its half-life in plasma is around 1 day. The peal concentration is achieved at 4 hours of drug administration.
- It is metabolized in liver. Its main metabolite is 6-desmethyl griseofulvin. Around 50% get excreted in urine as metabolites and 36% get excreted in feces.
Adverse Effects of Griseofulvin
- Common mild reactions include headache, GI disturbances (nausea, vomiting, flatulence, heart burn) and rashes. It may cause allergic and photosensitive reaction and share cross- sensitivity with penicillin.
- More serious effects include hepatotoxicity, hematologic effects including leucopenia, neutropenia, gynecomastia, pigmentation of genitals. These all occur rarely. However, it is recommended to perform blood studies weekly during therapy.
- It may affect nervous system and cause vertigo, peripheral neuritis, psychomotor incoordination and blurred vision. It can trigger effects of acute porphyria by interfering with porphyrin metabolism.
Drug Interaction
- It induces hepatic microsomal enzyme and hence, can increase metabolism of drugs like warfarin and oral contraceptives.
- Concurrent administration with phenobarbitone may retard its absorption from GI tract. It causes antabuse like reaction with alcohol.
Contraindication
- It is contraindicated in pregnancy.
- Contraindicated in patients allergic to penicillin. Contraindicated in patients with porphyria and hepatic insufficiency.
- It should not be used with alcohol.
- Its safety and efficacy had not been established in children 2 years of age and younger.
References
- https://www.ncbi.nlm.nih.gov/books/NBK548177/
- Lin CC, Magat J, Chang R, McGlotten J, Symchowicz S. Absorption, metabolism and excretion of 14c-griseofulvin in man. Journal of Pharmacology and Experimental Therapeutics. 1973; 187(2): 415-422.
- Fleece D, Gaughan JP, Aronoff SC. Griseofulvin Versus Terbinafine in the Treatment of Tinea Capitis: A Meta-analysis of Randomized, Clinical Trials. Pediatrics. 2004; 114 (5): 1312-1315.
- Alkeswani A, Cantrell W, Elewski B. Treatment of Tinea Capitis. Skin Appendage Disord. 2019; 5: 201–210.
- Lippincott Illustrated Reviews Pharmacology. 6th edition.
- Pharmacology and pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.