- Insomnia is one of the most prevalent sleep disorders that affects people of various age groups. It is characterized by difficulty in sleep initiation, difficulty in staying asleep, disturbed sleep due to nightmares. Insomnia has very deleterious effect on quality of life of patients as they experience daytime fatigue and decreased cognitive, social and physical functioning. Insomnia is also implicated in suicidal intention, Alzheimer’s disease and mood disorders. So, effective treatment for insomnia is required to have long-term improvement in quality of life beyond improved sleep.
- There are both non-pharmacological and pharmacological treatment of insomnia. Non-pharmacological treatment consists of behavioral approaches and cognitive behavioral therapy (CBT-I). The FDA approved pharmacological treatment of insomnia are benzodiazepines, non-benzodiazepine receptor agonists (Non-BzRAs), melatonin agonists, some tricyclic antidepressants and over the counter antihistamines. These medicines have restricted efficacy.
- Hypnotics with short half-life fails to maintain sleep for entire sleep duration whereas those with long half-life are associated with next-day hangover effects, increased risk of falls and motor vehicle accidents. Hypnotics acting on GABA (Gamma Amino Butyric Acid) receptor have adverse effects like hallucination, memory disturbance, somnolence. Non-BzRAs, such as zolpidem and eszopiclone have increased risk for complex sleep-related behaviors such as sleepwalking and sleep eating. The chances of reappearance of insomnia with discontinuation and bedtime physical and psychological dependence are also things to be considered. Orexin receptor antagonist are new category of drugs which have potential for effective treatment of insomnia. They are expected to promote sleep with less side effects.
What are orexins and orexin receptor antagonist?
- Orexins which are also known as hypocretins, are secreted from orexin containing neurons in lateral hypothalamus. Orexin-A and orexin-B are two neurochemically distinct forms of orexin and are widely distributed throughout the central and peripheral nervous system. These two neuropeptides play important role in physiological processes like sleep-wake rhythm, feeding behavior, drug addiction, glucose metabolism and stress- adaptive responses. Orexin bind to two G-protein coupled receptors, orexin receptors type 1 and type 2 (OX1R and OX2R). Orexinergic signaling produce prominent effect on maintenance of wakefulness through continuous depolarizing effects in wake-promoting brain nuclei.
- As binding of orexin to either of orexin receptors promote wakefulness, antagonists of those receptors could induce opposite effect to produce sleep. Orexin receptor antagonist block the orexin receptor for an extended period and reduce wake-promoting actions of orexin thus resulting in longer sustained periods of sleep. There are two distinct classes of orexin receptor antagonists: selective orexin receptor antagonist (SORAs) and dual orexin receptor antagonists (DORAs). SORAs have a binding affinity for either OX1R or OX2R. DORAs acts on both orexin receptors and is more effective in sleep-promoting effects. Many DORAs have had success in their respective clinical trials, with one successfully obtaining FDA approval for treatment of insomnia in 2014.
Some dual orexin receptor antagonist (DORA) are:
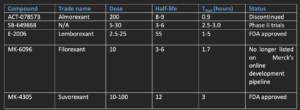
Almorexant
- It was developed in 2007 and was the first DORA to reach phase III clinical trials. Almorexant appears to be a competitive antagonist of OX1R and a noncompetitive antagonist of OX2R. In its preclinical and clinical trials, it had shown easy blood-brain barrier crossing capacity, induction of somnolence, improved sleep efficiency, reduction in time of sleep initiation and reduction of locomotor activity.
- Its clinical advancement was discontinued in 2011 because of safety concerns related to abnormal increase in liver enzyme concentration.
SB-649868
- It is orally administered DORA developed by GlaxoSmithKline and had shown improved sleep induction and sleep maintenance, reduced sleep latency in its clinical trials. It has shown minimum adverse effects and can be well tolerated with doses up to 80 mg.
Lemborexant
- Lemborexant acts as competitive antagonist of both OX1R and OX2R. It had shown improvement in mean sleep efficiency compared to placebo in phase II clinical trials. Side effects like sleep paralysis, somnolence and headache was reported. Phase III trials was conducted in 2018 in patients with general insomnia along with a phase II study testing lemborexant in patients with irregular sleep-wake rhythm disorder and dementia.
- It was approved for treatment of insomnia in adults in USA in 2019. It is available as 5 mg and 10 mg tablets. Lemborexant can be used in treatment of primary insomnia and insomnia associated with other diseases like depression.
Filorexant
- Filorexant (MK-6096) is a DORA developed by Merck and Co. with a slightly different chemical composition from that of almorexant. It had shown a favorable pharmacokinetic profile, higher bioavailability and more rapid binding to orexin receptors than almorexant at smaller dose. It has short half-life compared to other DORAs and thus have less residual effect.
- Somnolence is observed but only at doses above 10 mg. As of April 2020, filorexant is no longer listed on Merck’s online development pipeline.
Suvorexant
- Suvorexant, developed by Merck and Co., was 1st DORA to be approved by the FDA for treatment of insomnia. It was approved in August 2019. The dose range is 10–20 mg/day with a half-life of 12.2 hours. Existing data available on the safety profile of suvorexant is limited. So far, the medication has been well tolerated by elderly (age 65 years and older)71 and nonelderly (age 18–64 years) men and women with insomnia at doses up to 20 mg.
- In its clinical trials, it doesn’t show evidence of withdrawal, or rebound effects upon discontinuation of the medication, cognitive and motor impairments, next day hangover. The main side effect was somnolence seen in 6.7% of patients. Cataplexy (a medical condition in which strong emotion or laughter causes an individual to suffer sudden physical collapse) was not observed in patients administered a therapeutic dose although it has occurred at higher doses. Therefore, patients treated with a dual orexin antagonist might be expected to be associated with cases of cataplexy.
Table- Overview of Dual orexin receptor antagonists (DORAs)
Comparison of orexin receptor antagonists with currently available sedative hypnotics
- It is difficult to determine exact comparison of dual orexin receptor antagonists with currently available sedative hypnotics without head-to-head trials. DORAs offers an alternative approach in treating insomnia by acting on orexin receptor. As orexin signaling plays important roles in many other physiological processes, it may show more side effect profile.
- Currently, there is a knowledge gap about rarer side effects of DORAs and potential drug interactions with first line insomnia treatment. The increased cost of suveraxant in comparison with other hypnotics is also a subject of concern.
- According to some recent studies, orexin potentially mediates the relationship between disturbed sleep and the pathogenesis of Alzheimer’s disease (AD) via modulation of ISF (brain interstitial fluid) Aβ concentrations. Because of this newly established role of orexin in AD, and the bidirectional relationship between disturbed sleep and AD progression, DORAs may offer a potential treatment of comorbid insomnia in patients with AD. More clinical trials are needed to evaluate the effectiveness of DORAs for the treatment of not only primary insomnia, but also in secondary insomnia with other medical comorbidities.
References
- Calhoun SL, Fernandez-Mendoza J, Vgontazas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med. 2014; 15(1): 91–95.
- Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011; 69(6): 592–600.
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989; 262(11): 1479–1484.
- Morin CM, Benca R. Chronic insomnia. Lancet. 2012; 379(9821): 1129–1141.
- Jaussent I, Empana JP, Ancelin ML, et al. Insomnia, daytime sleepiness and cardio-cerebrovascular diseases in the elderly: a 6-year prospective study. PLoS One. 2013; 8(2): e56048.
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015; 163(3): 191–204.
- Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016; 165(2): 125–133
- Pinto LR, Jr, Bittencourt LR, Treptow EC, Braga LR, Tufik S. Eszopiclone versus zopiclone in the treatment of insomnia. Clinics (Sao Paulo) 2016;71(1):5–9.
- https://www.sleepfoundation.org/sleep-aids/orexins