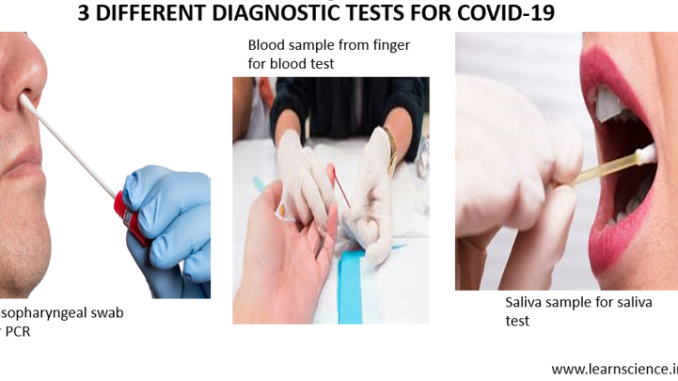
There are currently two main tests for the COVID-19 detection; the rapid test (for antibody blood test) and PCR test. On Tuesday, April 13, 2020 FDA had approved new saliva test as ‘emergency test’ for detection of COVID-19.
Difference between PCR swab test and antibody blood test

The New Saliva Test
- On April 13, 2020, FDA has approved the use of saliva samples as a means of emergency testing for COVID-19. This test was developed at Rutgers University, USA and is not currently approved for home administration.
- The saliva is tested for the presence of viral genetic material by RT-PCR.
- This involves taking saliva sample in a tube (spitting in a tube) which is less invasive than nasopharyngeal swab. It also decreases the risk among health care workers who had to come in close contact with COVID-19 patients to take their nasopharyngeal swab.
References
- https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19.
- https://www.fda.gov/media/134922/download.