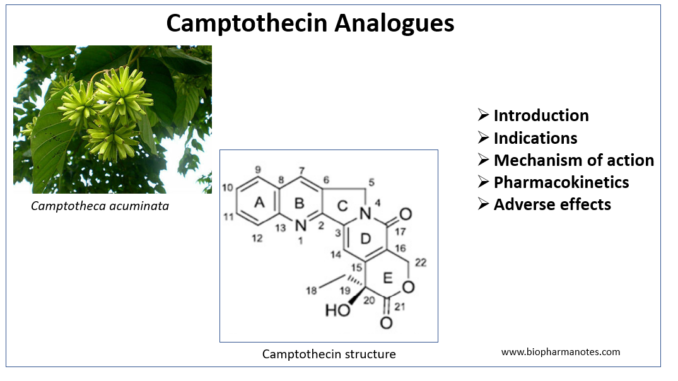
- Camptothecin (CPT) are cytotoxic quinoline alkaloids which are originally isolated from Chinese tree Camptotheca (Camptotheca acuminata). Camptothecin analogues like topotecan and irinotecan are used to treat different kind of cancer.
- Wall ME and Wani MC were the first to isolate camptothecin from bark of Camptotheca tree in 1966. During that time, due to low stability and solubility of camptothecin it’s use was not preferred. However, in late eighties when molecular target of CPT was identified, it became popular among researchers. FDA approved two camptothecin analogues; topotecan and irinotecan in beginning of 20th century. Recently approved CPT analogue includes transtuzumab derutexan.
- The structure consists of a pentacyclic ring structure which begins with weakly basic quinoline moiety and ends with a lactone ting.
- Irinotecan is included in World Health Organization’s List of Essential medicines.
Indications of Camptothecin Analogues
- They are effective in treating lung, ovarian, breast, stomach and pancreatic cancer.
- Topotecan is used as secondary agent for treating ovarian cancer and small cell lung cancer. Irinotecan is used as primary agent with 5 fluorouracil in treatment of metastatic colorectal cancer.
Mechanism of action of Camptothecin Analogues
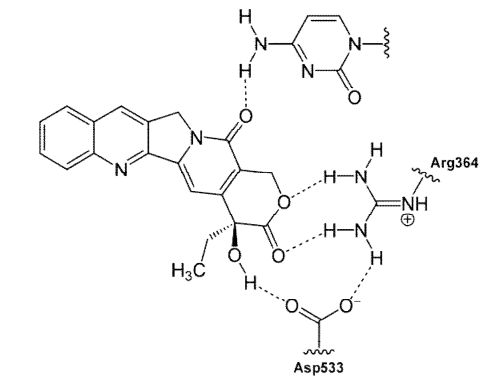
Figure- Binding of camtothecin to DNA and topoisomerase I (Source- Muqeet et al, 2014)
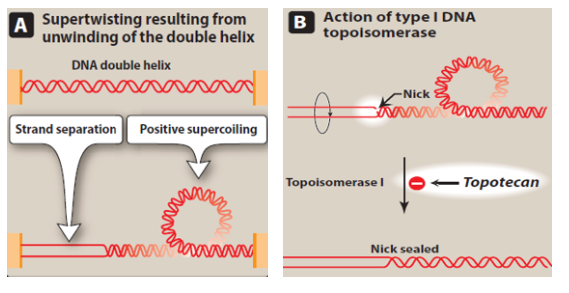
Figure- Mechanism of action of campothecin analogues (Source- Lippincott’s Illustrated Reviews)
- They bind to nuclear enzyme topoisomerase 1st and inhibit DNA replication. DNA topoisomerase are enzymes required for cutting and ligation of DNA strand. Topoisomerase 1st bind covalently to double stranded DNA and produce single strand DNA break leading to uncoiling of DNA. Once the DNA torsional strain is relieved, the break is resealed by topoisomerase I.
- The drug mainly affects ligation of DNA by topoisomerase and hence lead to accumulation of single strand breaks in DNA which results in cell death by causing irreversible double strand DNA break. They are S phase specific.
Pharmacokinetics of Camptothecin Analogues
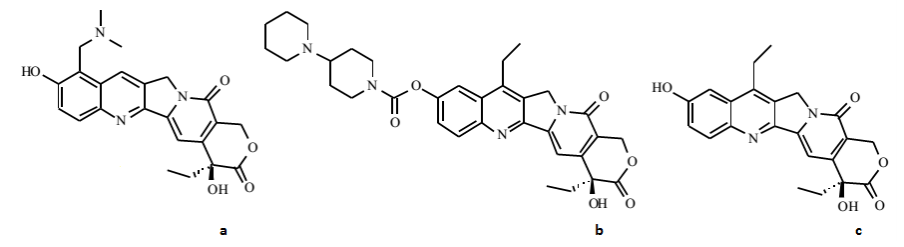
Figure – Structure of a) Topotecan b) Irinotecan and c) SN-38. (source- Muqeet et al, 2014)
- Irinotecan is a prodrug which is converted to active metabolite SN-38 (7-ethyl-10-hydroxy-camptothecin) by carboxylesterase. Only a small fraction of irinotecan is converted to its active form. The biological half-life of SN-38 is around 11.5 hours. Plasma protein binding of irinotecan is 30-43% and for SN-38 is 92-96%. Irinotecan is also converted to two other metabolites which are poor inhibitors of topoisomerase 1st. It is mainly excreted via biliary route.
- Topotecan is mostly administered as 30 minutes IV infusion. Around 20-30% of drug is found in active lactone form in plasma. Its biological half-life is 3.5-4.1 hours. Plasma protein binding is low around 7-35% which is the reason behind its greater CNS penetration. It is mainly excreted through renal excretion in form of urine.
Adverse Effects
- Topotecan can cause bone marrow suppression especially neutropenia. Patients taking this drug should be monitored frequently for blood count.
- Irinotecan may cause myelosuppression and severe delayed diarrhea (due to inhibition of acetylcholinesterase activity). Febrile neutropenia associated with concomitant diarrhea may be fatal. Severe diarrhea can be treated by suing atropine during infusion or high doses of loperamide in days following the infusion.
References
- Wahid M, Bano Q. Camptothecin and its analogs antitumor activity by poisoning topoisomerase I, their Structure activity relationship and clinical development perspective of analogs. J App Pharm Vol. 2014; 6(3): 286 -295.
- Carbnero RG, Supko JG. Current Perspectives on the Clinical Experience, Pharmacology, and Continued Development of the Camptothecins. Clinical Cancer Research. 2002; 8(3): 641-661.
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8405929/