- Atomoxetine is selective nor- epinephrine reuptake inhibitor (SNRI) used in management of attention deficit hyperactivity disorder (ADHD). (ADHD) is a condition characterized by high level of impulsivity, hyperactivity, and inattention. It is one of the most common neurodevelopmental disorder occurring in childhood. The symptoms of ADHD may begin in childhood and may continue up to teenage and adolescence.
- It was approved by FDA for treatment of ADHD in 2002 and is also available as generic medicine.
- It is first non-stimulant agent approved by FDA for ADHD.
Indications of atomoxetine
- Its FDA approved use is treatment of ADHD in adolescents, adults and children over age of 6 years.
- Its off-label use is management of treatment resistant depression in adult patients.
Mechanism of action of atomoxetine
- Though the pathophysiology of ADHD is not clear, certain research suggests that imbalance in dopaminergic and nor- adrenergic pathway in prefrontal regions of brain plays important role in its pathophysiology.
- It is potent and selective inhibitor of norepinephrine transporter (NET) which prevents cellular reuptake of norepinephrine and resulting in increased concentration of intrasynaptic norepinephrine in brain. It also causes increase in extracellular concentration of dopamine in prefrontal cortex of brain, but not in striatum or nucleus accumbens.
- Some studies had also indicated its effect in serotonin transportation. More research is required in this for detailed results.
Pharmacokinetics of atomoxetine
- It is administered via oral administration. Available as hydrochloride salt i.e. atomoxetine HCl. It is available in the form of 10 mg, 18 mg, 25 mg, 40 mg, 60mg, 80 mg and 100 mg capsules.
- It’s absorption is rapid after oral administration. It is administered with or without food. Its volume of distribution is 1.6- 2.6 L/kg. It has good protein binding properties, especially to albumin. It reaches to maximum plasma concentration after around 1-2 hours of administration of oral single dose.
- Metabolism takes place by CYP2D6 enzymatic pathway. The major metabolite is 4 hydroxy atomoxetine which have similar potency as of atomoxetine. 4 hydroxy atomoxetine undergoes further glucuronidation. Atomoxetine is also metabolized by CYP2C19 to N- Desmethyl- Atomoxetine which is not as potent as atomoxetine.
- Its metabolite 4-hydroxyatomoxetine-O-glucuronide is excreted mainly via urine and small amount is excreted via feces. Its half-life is around 3-5.6 hours.
- As it undergoes extensive hepatic metabolism, dosage adjustment is required in patients with severe hepatic impairment.
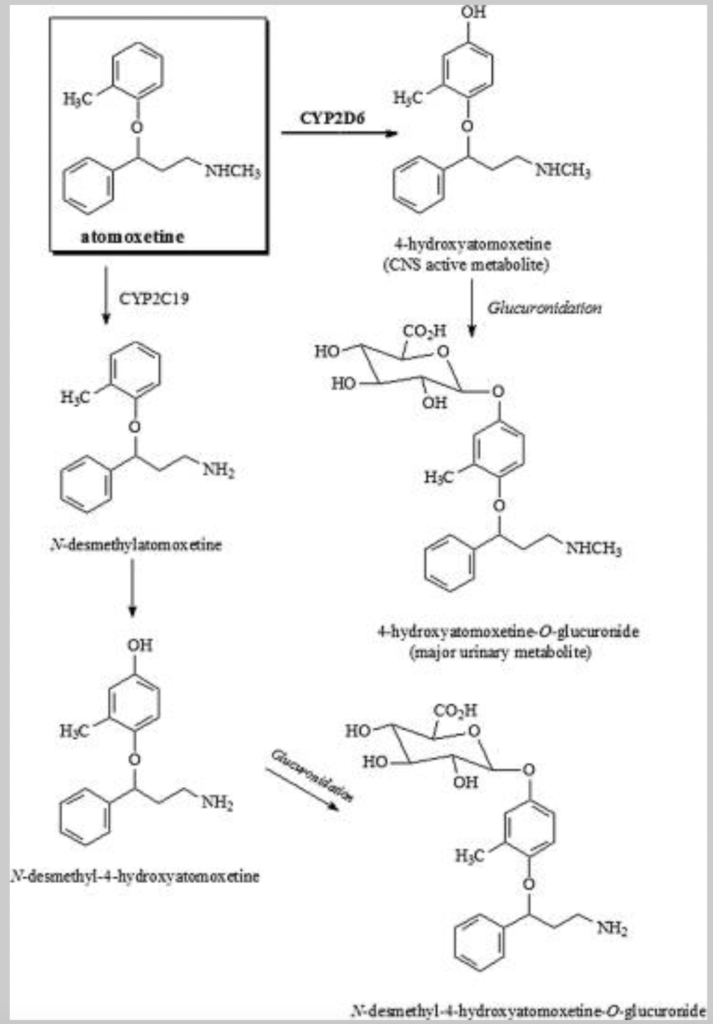
Figure- Metabolites of Atomoxetine in Human (Source- Yu G et al, 2016)
Adverse effects
- It has black box warning for increasing suicidal intention and thoughts in children and adolescents. Any individual taking atomoxetine should be monitored for unusual changes in behavior and suicidal ideation. The chances of having depression increases by around 5 times in patients taking atomoxetine compared to normal population.
- Other side effects include insomnia, headache, erectile dysfunction, constipation, xerostomia and abdominal pain. It can also cause flushing, syncope, orthostatic hypotension, increased thirst, urinary retention, prostatitis etc.
Drug Interactions
- Potent and selective CYP2D6 inhibitors like paroxetine, fluoxetine etc can cause increase in side effects of atomoxetine by inhibiting its metabolism and increasing its concentration. Hence, dosage adjustment of atomoxetine is needed during concurrent administration with these agents.
- It may interact with other nor-epinephrine reuptake inhibitors and MAOIs (Mono amine oxidase inhibitors).
Contraindication
Contraindicated in following conditions
- Patients allergic to atomoxetine or other ingredients of its formulation.
- Patient with history of pheochromocytoma.
- In arrow angle glaucoma.
- In cardiac patients especially those whose medical condition could be worsened by increase in BP or heart rate is highly risky.
- It should not be used if the patient is on MAOI (Mono amine Oxidase Inhibitor) therapy within last 14 days.
- Its use in pregnancy and breastfeeding is not well studied.
Comparison with stimulants used for ADHD
- Stimulants like methylphenidate, dextroamphetamine and lisdexamfetamine are used for treatment of ADHD. These drugs increase dopamine in subcortical nucleus accumbens and striatum which leads to many stimulant associated side effects and increase in drug abuse. As atomoxetine increases dopamine level only in prefrontal cortex, it is free of such side effects and potential for drug abuse.
References
- https://www.statpearls.com/ArticleLibrary/viewarticle/17954
- https://go.drugbank.com/drugs/DB00289
- Yu G et al. Atomoxetine: A Review of Its Pharmacokinetics and Pharmacogenomics Relative to Drug Disposition. J Child Adolesc Psychopharmacol. 2016 May 1; 26(4): 314–326.
- Sauer JM et al. Clinical Pharmacokinetics of Atomoxetine. Clinical Pharmacokinetics. 2005; 44: 571-590.
- Clemow DB et al. A review of the efficacy of atomoxetine in the treatment of attention-deficit hyperactivity disorder in children and adult patients with common comorbidities. Neuropsychiatric Disease and Treatment. 2017:13 357–371.