- Dapagliflozin represent the class of drugs SGLT-2 inhibitors (Sodium Glucose Co-transporter-2 inhibitors). SGLT-2 inhibitors are newer class of oral hypoglycemic agents. Dapagliflozin got its FDA approval on 2014 and is available as different brands like Forxiga, farxiga, Qtern, Xigduo etc.
Indications of Dapagliflozin
- It is approved for use in Type 2 diabetes along with diet and exercise.
- To reduce risk of hospitalization due to heart failure in adults with type 2 diabetes and cardiovascular disease or other risk factors.
- It is recently approved by FDA for its use on heart failure patients with reduced ejection fraction (HFrEF) with and without type 2 diabetes. Astrazeneca’s Farxiga was the one which got FDA approval on May 2021.
Mechanism of action of Dapagliflozin
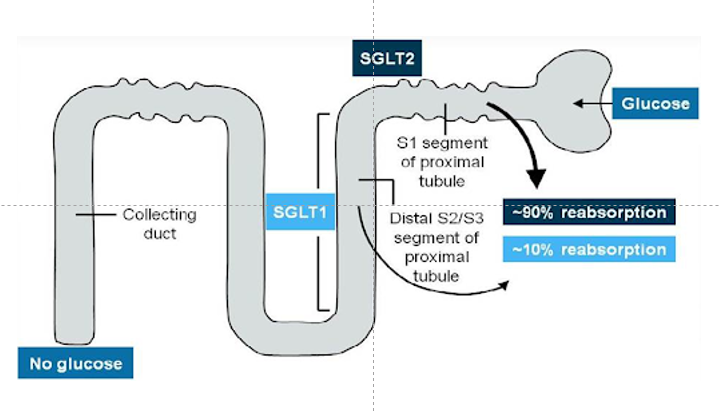
Figure- SGLT 2 play major role in glucose reabsorption in kidney.
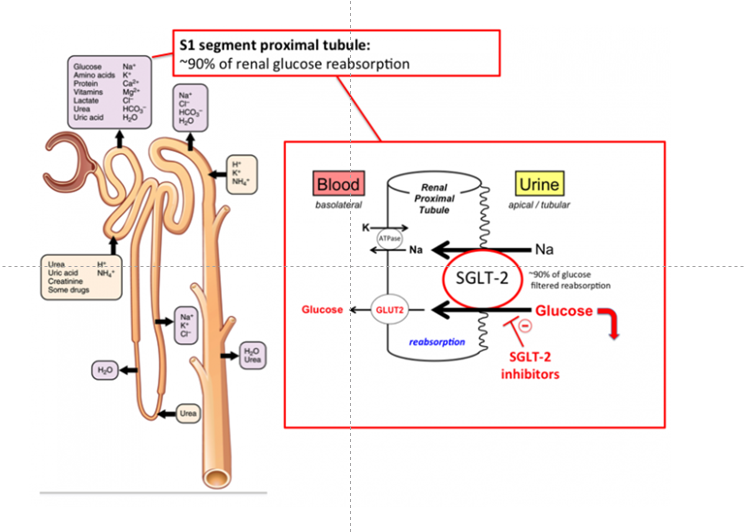
Figure- Mechanism of action of Dapagliflozin (Source- tmedweb.tulane.edu)
- Dapagliflozin is orally active, reversible, and highly selective SGLT-2 inhibitors.
- SGLT-2 plays important role in glucose and sodium reabsorption in nephron of kidney. Plasma glucose is freely filtered by kidney, but none appears in urine. It’s because all the filtered glucose are normally reabsorbed. Bulk of glucose reabsorption takes place in S1 and S2 segment and remaining in S3 segment of nephron. There are 6 known SGLT type in human body among which type 1, and type 2 are most abundant. SGLT-2 found in S1 segment of proximal tubule is responsible for 90% glucose reabsorption within kidney. SGLT-1 found in S2/S3 segment and responsible for 10% of glucose reabsorption within kidney.
- One of the major symptoms of type 2 diabetes is hyperglycemia. Hyperglycemia causes upregulation of SGLT-2 proteins which increases glucose reabsorption and further causes hyperglycemia. However, after certain time as capacity of nephron is overwhelmed, leads to glycosuria.
- Dapagliflozin, like other SGLT-2 inhibitors inhibit SGLT-2 and hence decrease reabsorption of glucose, increase urinary glucose excretion leading to lowering of body glucose level.
Pharmacological effects of dapagliflozin
- It increases urinary glucose excretion. It enhances insulin sensitivity and have effect on glucose metabolism by increasing glucagon level. Glucagon level is increased due to increase in expression of glucagon gene and reduction of HNF4A (Hepatocyte Nuclear factor 4 a).
- It has beneficial effect on blood pressure, body weight and lipid parameters. So, it is recommended in type 2 diabetic patients who need to lose body weight. Dose dependent reduction of uric acid level is also one of the effect of dapagliflozin.
- Recent trials like DAPA-HF (Dapagliflozin and Prevention of Adverse outcomes in Heart failure) had proved the efficacy of dapagliflozin in patients with heart failure with reduced ejection fraction (HFrEF). The major mechanism involved is not known and ye to be elucidated.
Pharmacokinetics
- It is orally administered and reaches peak plasma concentration within 1.5- 2 hours. Its efficacy is not affected by food consumption. Metabolism takes place in liver and kidney. Uridine diphosphate glucuronosyltransferase play major role in its metabolism. Dapagliflozin undergoes glucuronidation and form metabolite 3-O-glucuronide (which is inactive).
- It has large volume of distribution (118L) and long half-life of 12.5 hours. Hence, it is administered as single dose regimen. Route of elimination includes both urinary and fecal excretion. Around 75% of administered dapagliflozin is excreted in urine and 21% is excreted via feces.
Adverse effects
- Common side effects include female genital mycotic infections, urinary tract infections and urinary frequency. Some other adverse effects are diabetic ketoacidosis, orthostatic hypotension and increase in creatinine level.
Contraindications
- It should not be used in type 1 diabetic patients and in diabetic ketoacidosis.
- It is not used in patients with creatinine clearance below 45 mL/min and is contraindicated in patients with creatinine clearance below 30 mL/min.
- Not recommended in second and third trimester of pregnancy.
References
- https://go.drugbank.com/drugs/DB06292
- https://pharmacia.pensoft.net/article/70626/
- Shah KS etal. Annu. Rev. Pharmacol. Toxicol. 2022. 62: 109–20.
- Papakitsou I et al. Differential pharmacology and clinical utility of dapagliflozin in type 2 diabetes. 2019; 11: 133-143.
- Vivian EM et al. Dapagliflozin: A new sodium–glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. American Journal of Health-System Pharmacy. 2015; 72 (5): 361-372.
- Dhillon S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs. 2019; 79(10): 1135–1146.