- Flumazenil is imidazo- benzodiazepine which is used as benzodiazepine antagonist. It is a selective GABA-A receptor antagonist.
- It was first marketed in 1987 and was FDA approved in 1991. Nowadays, it is available both as brand and generic medicine.
Therapeutic Uses of Flumazenil
- To reverse benzodiazepine (BDZ) anesthesia– Patient anesthetized with BDZ when injected with 0.3- 1 mg of flumazenil can wake up and regain motor control within 1 minutes. Hence, it is used in early discharge of patients after diagnosis or surgery and is helpful in post anesthetic management.
- In BDZ poisoning or overdose- used in suspected case of BDZ overdose. It may help in rapid return of consciousness within 5-15 minutes of injection. In most of the cases of BDZ overdose, patients will respond to cumulative dose of 1-3 mg. In rare cases, additional dose up to 5 mg may be required. If there is no response after 5minutes of cumulative 5 mg dose, the main cause of sedation may not be benzodiazepines.
- Some of its non-FDA approved uses are-
- Alcohol withdrawal syndrome
- In reversing drug action of Baclofen
- In toxicity of drug cannabis
Mechanism of action of Flumazenil
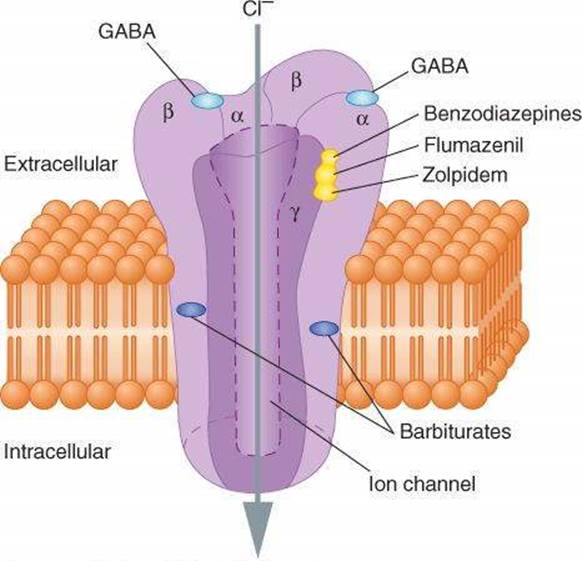
Figure 1- Binding sites of BDZ and Flumazenil ( Source- Katzung and Trevor’s Pharmacology)
- It binds competitively to BDZ binding site and antagonizes the allosteric action of BDZ and other ligands. Flumazenil can antagonizes both behavioral and electrophysiological effects of BDZ. It doesn’t block pharmacological effects of GABA or GABA-mimetics.
- It also blocks effects of non-benzodiazepine agonists like zopiclone at BDZ receptor.
Pharmacokinetics of Flumazenil
- It is mostly given by IV injection. The initial dose is 0.2 mg over 30 seconds followed by 0.3 mg every minute to a total of 3 mg. IV infusion can also be used. When administered through oral route, it is rapidly absorbed and undergoes extensive first pass metabolism. So, it is not used orally.
- It has rapid onset of action and short duration of action. Its half-life is around half an hour. To reverse effect of long acting benzodiazepines like diazepam and quazepam, frequent administration may be required.
- It is metabolized in liver and is eliminated almost entirely by hepatic metabolism.
Adverse effects
- It may cause agitation, discomfort, coldness and dizziness. If used in patients dependent on benzodiazepine, it may cause withdrawal symptoms. If used in patients who use BZD for seizures, it may precipitate seizures.
- It mayalso precipitate seizures in patients poisoned with tricyclic antidepressants or antipsychotics.
Contraindications and warnings
- It should be used with caution in poisoning due to mixed drugs as it may produce serious adverse effects if used in patients poisoned with tricyclic antidepressants or antipsychotics.
- Contraindicated in epileptic patients taking BDZ for controlling seizures.
- It is FDA pregnancy category C drugs. Its effect on fetus is not known. It is not known whether flumazenil passes into breast milk and harm baby or not. So, a patient should inform doctor, if she is pregnant or breastfeeding.
- Contraindicated in patients hypersensitive to flumazenil or BDZ.
- Patients should be monitored for possible return of sedation. Monitoring for respiratory depression and other residual effects of BDZ should be done for around 2 hours.
References
- Pharmacology and Pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.
- Lippincott Illustrated Reviews Pharmacology, 6th edition.
- Essentials of Medical Pharmacology. 7th edition.
- https://www.statpearls.com/articlelibrary/viewarticle/21842/