
Introduction of orphan drugs
- Orphan drugs are medicines or vaccines intended to treat, prevent or diagnose rare medical condition referred as ‘orphan disease’. Such rare diseases include genetic diseases, rare cancers, infectious tropic diseases and degenerative diseases.
- The definition of rare disease may vary according to country. In the United States rare diseases are defined as a disease or a condition which affects fewer than 200,000 patients in the country. According to the European Union (EU) rare disease are defined as a life-threatening or chronically debilitating condition affecting no more than 5 in 10,000 people.
- About 80 % of these diseases are caused due to genetic reasons and some others are caused due to environmental, bacterial, viral or unknown origin.
- Every year, about 250 new rare cases or diseases are reported. Around 6000–8000 rare diseases are identified till today, affecting approximately 6–8% of the world’s population. However, there are treatments available only for 200-300 orphan diseases.
- Orphan drugs are developed by pharmaceutical industries not for economic reasons but to meet public health demand.
- Orphan drugs are not easily available because their production is not commercial-viable due to various reasons like;
– Limited demand.
– Huge cost of production.
– Non-patentability of the drug.
– The complex and costly procedure for establishing efficacy and safety of drug and government approval process.
- Despite the need and importance of orphan drugs, modern medical society is facing their scarcity. This scarcity is due to high cost in developing drugs with less profit potential which is again due to small number of patients per rare disease indication.
- Orphan drugs are expensive often exceeding €100,000 per patient per year.
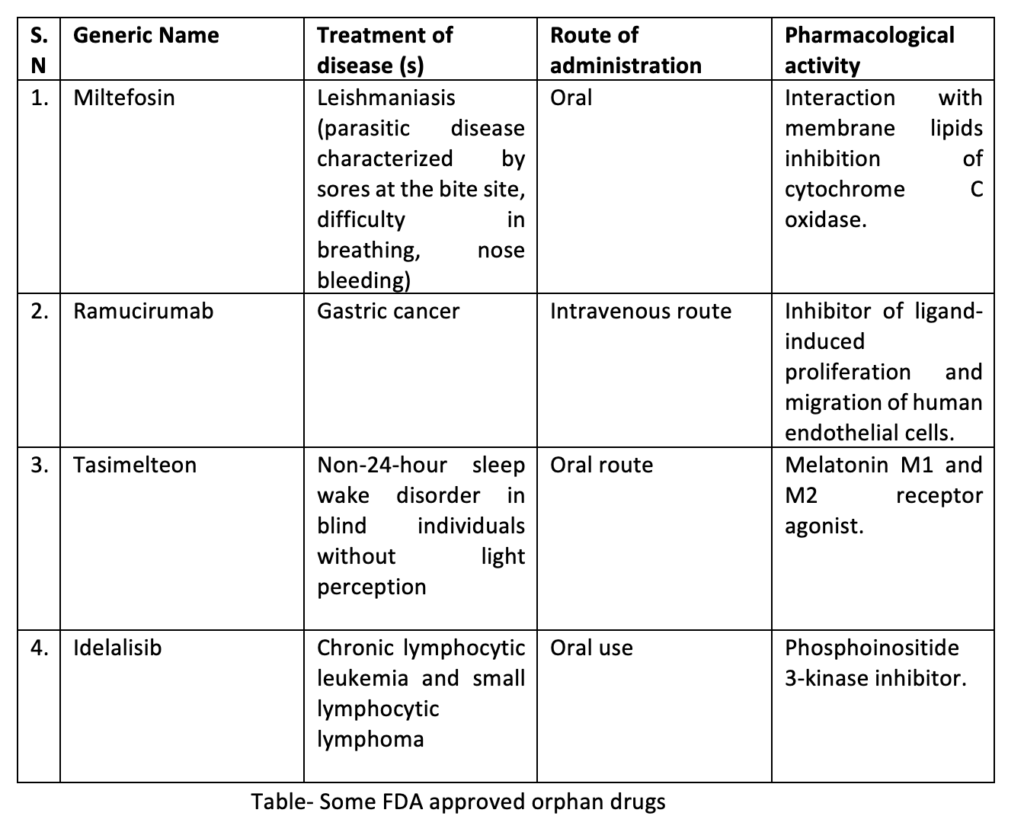
Orphan Drug Policy
- Many governments and authorities have established legislations, regulations and policies to encourage the research and development of orphan drugs and to address licensing regulations and pricing and reimbursement of these drugs.
- Many developed countries such as the US, Japan, Australia, and European countries have established a range of legislations or policies for orphan drugs. However, many Asian countries fall behind in this field. A few Asian countries such as Japan, Singapore and Taiwan have made developments in this area.
- US was the first country to establish national orphan drug legislation with the Orphan Drug Act passed in 1983. Japan was the second country to implement orphan drug legislation in 1993.
- The main purpose of these legislation is to encourage orphan drug research, production and marketing by providing financial and non-financial incentives. Financial incentives may include providing research grants, tax credits, corporate tax reduction, marketing exclusivity and user fee waivers. Non-financial incentives include free scientific advice, protocol assistance, fast priority review for marketing authorization and pre-licensing access initiatives.
- Pre-licensing allows import of orphan drugs from other countries but currently unauthorized in the country. It is most common methods for rare disease patients to get access to orphan drugs in many countries. Pre-licensing allows use of orphan drugs by an individual or group of patients suffering from serious or life-threatening disease when there is no alternative therapeutic option.
- Marketing exclusivity allows pharmaceutical companies several years to recover costs for drug development. During the marketing exclusivity period, regulatory agencies cannot approve a generic drug or brand name drug for use in same rare disease. However, the same drug can receive approval for use in some other disease.
Reference
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4599885/
- https://www.sciencedirect.com/science/article/pii/S1359644618304380
- https://www.imedpub.com/articles/recent-research-on-orphan-drugs-an-overview.pdf
- Pharmacology and Pharmacotherapeutics. 24th edition.