• Clonidine is an imidazoline derivative having wide therapeutic application.
• Clonidine was synthesized in 1962 as derivative of known alpha sympathomimetic drug naphazoline and came into medical use in 1966. It is available as generic medicine.
Mechanism of action of clonidine
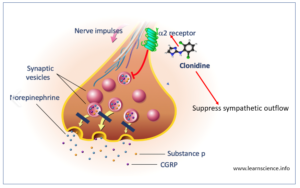
Figure 1- Mechanism of action of Clonidine
• It is alpha sympathomimetic drug which acts centrally as an α-2 agonist.
• It acts on adrenergic α-2 receptors present in vasomotor center and hypothalamus and activate them. Activation of these receptors decrease release of nor-adrenaline from nerve terminal and suppress sympathetic outflow to periphery resulting in decreased blood pressure.
• It also acts on imidazoline receptors in brain resulting in its anti-hypertensive action.
• In large doses, it stimulates peripheral inhibitory pre-synaptic alpha-2 receptors and decrease peripheral NA release.
Pharmacological Actions of clonidine
• When administered through IV route, it produces transient hypertensive response followed by long-term decrease in both systolic and diastolic BP. Initial hypertensive effect is not seen with oral administration.
• It reduces both supine and standing BP. It decreases heart rate.
• The hypotensive action is also associated with reduced cardiac output, decreased total peripheral resistance or both. The reduction in cardiac output become less apparent with time.
• It reduces cerebral blood flow, cerebral metabolic rate of oxygen consumption and intraocular pressure. It produces dose dependent sedation, analgesia and anxiolytic activity.
• It decreases plasma renin activity.
Pharmacokinetics of clonidine
• It is available as oral tablet, transdermal patches and injection for epidural infusion. Topical gel formulation is also available.
• It is highly lipid soluble so is well absorbed from gut with bioavailability around 100%. The peak plasma concentration and maximum hypotensive effect are observed 1-3 hours after oral administration.
• When applied as transdermal patch, 3-4 days are required to reach steady state plasma concentration. After removal of patch, plasma concentration remains stable for around 8 hours and decline gradually over several days.
• Due to its high lipid solubility, it can penetrate CNS easily. It is metabolized in liver to inactive metabolites.
• The elimination half-life of drug ranges from 6-24 hours (mean ̴12 hours).
• About half of the administered drug is excreted unchanged in urine. The half-life of drug may increase in case of renal failure. About 20 % of drug is excreted in feces.
Therapeutic Uses
• In hypertension. However, its use as anti-hypertensive agents had declined nowadays. It is used only in exceptional cases.
• In menopausal hot flushes. Transdermal patches are useful.
• Some of its off-label uses are:
– In nicotine, opiate and alcohol withdrawal. It helps in counteracting withdrawal symptoms, which are caused due to catecholamine release, including pupillary dilation, nausea, vomiting, lacrimation, piloerection, diarrhea and anorexia.
– In attention deficit-hyperactivity disorder (ADHD).
– In Tourette’s syndrome.
– Used as an adjuvant to intrathecal local anesthetics and to improve intraoperative analgesia and to increase duration of sensory and motor block.
– For reducing diarrhea in some diabetic patients with autonomic neuropathy.
– In diagnosis of growth hormone deficiency.
– Prophylaxis of migraine.
– In dysmenorrhea and certain anxiety disorder.
– As a sedative agent in intensive care unit (ICU).
Adverse Effects
• The major adverse effects are dry mouth and sedation which decreases after several weeks of therapy. Other common side effects are drowsiness, constipation, GI disturbances, dry eye, vertigo and miosis.
• It may cause bradycardia and sexual dysfunction in high doses. These effects are less with transdermal administration.
• Dermatitis or allergic reaction occur in case of transdermal patches.
• Sudden cessation of long-term clonidine therapy may cause hyperirritability and dangerous and lethal rebound increase of BP. So, discontinuation should be done cautiously, and slow titration should be performed.
Drug Interaction
• It may interact with drugs like atenolol, propranolol, amlodipine, ibuprofen, losartan, prazosin, sildenafil (Viagra), diazepam and alprazolam.
• Clonidine and ethanol may have additive effects in lowering of BP.
Contraindication
• Not recommended in pregnancy and breastfeeding.
Reference
- https://www.drugs.com/drug-interactions/clonidine.html
- https://www.hartleymedical.com/pharmacology-a-review-clonidine/
- https://anesthesiageneral.com/clonidine/
- Elia N, Culebras X, Mazza C, Schiffer E, Ramer MR. Clonidine as an Adjuvant to Intrathecal Local Anesthetics for Surgery: Systematic Review of Randomized Trials. Regional Anesthesia and Pain Medicine. 2008; 33(2): 159-167.
- Jamadarkhana S, Gopal S. Clonidine in Adults as a Sedative Agent in the Intensive Care Unit. J Anaesthesiol Clin Pharmacol. 2010; 26(4): 439–445.
- Naguy A. Clonidine Use in Psychiatry: Panacea or Panache? 2016; 98: 87–92.
- Musini VM, Pasha P, Gill R, wright JM. Blood pressure lowering efficacy of clonidine for primary hypertension. Cochrane Database Syst Rev. 2017; (9): CD008284.
- Pharmacology and pharmacotherapeutics. 24th edition.
- Goodman and Gillman Manual of Pharmacology and Therapeutics.